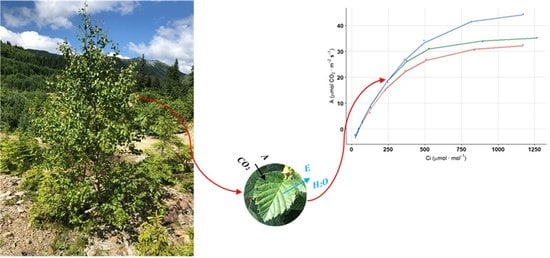
Photosynthesis Traits of Pioneer Broadleaves Species from Tailing Dumps in Călimani Mountains (Eastern Carpathians)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Gas Exchange Measurements
2.3. The Farquhar–Von Caemmerer–Berry Model
2.4. Data Analyses
3. Results and Discussion
3.1. Photosynthetic Parameters for Deciduous Pioneer Species
3.2. Variability of Photosynthetic Parameters during the Vegetation Season
3.3. Photosynthesis Response Curve under Increasing CO2 Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.R.; Skea, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. IPCC, 2019: Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019; pp. 1–864. [Google Scholar]
- Liu, S.; Li, W.; Qiao, W.; Wang, Q.; Hu, Y.; Wang, Z. Effect of Natural Conditions and Mining Activities on Vegetation Variations in Arid and Semiarid Mining Regions. Ecol. Indic. 2019, 103, 331–345. [Google Scholar] [CrossRef]
- Chauhan, S.S. Mining, Development and Environment: A Case Study of Bijolia Mining Area in Rajasthan, India. J. Hum. Ecol. 2010, 31, 65–72. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Zhou, Y.; Zhu, W.; Yin, Y. Variations of Soil Microbial Communities Accompanied by Different Vegetation Restoration in an Open-Cut Iron Mining Area. Sci. Total Environ. 2020, 704, 135243. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.F.; Burken, J.G.; Maier, R.M.; Newman, L.A.; Rock, S.; Schnoor, J.L.; Suk, W.A. Phytotechnologies—Preventing Exposures, Improving Public Health. Int. J. Phytoremediat. 2013, 15, 889–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest Restoration Following Surface Mining Disturbance: Challenges and Solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef] [Green Version]
- Pietrzykowski, M. Tree Species Selection and Reaction to Mine Soil Reconstructed at Reforested Post-Mine Sites: Central and Eastern European Experiences. Ecol. Eng. X 2019, 3, 100012. [Google Scholar] [CrossRef]
- Frouz, J.; Dvorščík, P.; Vávrová, A.; Doušová, O.; Kadochová, Š.; Matějíček, L. Development of Canopy Cover and Woody Vegetation Biomass on Reclaimed and Unreclaimed Post-Mining Sites. Ecol. Eng. 2015, 84, 233–239. [Google Scholar] [CrossRef]
- Cenușă, E. Instalarea Vegetației Naturale În Zone Afectate de Activități Miniere Din Parcul National Călimani; Forestry Technical Publishing House: Voluntari, Romania, 2018. [Google Scholar]
- Boscutti, F.; Poldini, L.; Buccheri, M. Green Alder Communities in the Alps: Phytosociological Variability and Ecological Features. Plant Biosyst. 2014, 148, 917–934. [Google Scholar] [CrossRef]
- Mauri, A.; Caudullo, G. Alnus viridis in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; European Commission: Brussels, Belgium, 2016; p. 68. [Google Scholar] [CrossRef]
- Tenkanen, A.; Keski-Saari, S.; Salojärvi, J.; Oksanen, E.; Keinänen, M.; Kontunen-Soppela, S. Differences in Growth and Gas Exchange between Southern and Northern Provenances of Silver Birch (Betula Pendula Roth) in Northern Europe. Tree Physiol. 2020, 40, 198–214. [Google Scholar] [CrossRef]
- Beck, P.; Caudullo, G.; de Rigo, D.; Tinner, W. Betula pendula, Betula pubescens and Other Birches in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; European Commission: Brussels, Belgium, 2016; pp. 70–73. [Google Scholar] [CrossRef]
- Caudullo, G.; de Rigo, D. Populus tremula in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; European Commission: Brussels, Belgium, 2016; pp. 138–139. [Google Scholar] [CrossRef]
- Enescu, C.M.; Houston Durrant, T.; de Rigo, D.; Caudullo, G. Salix caprea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; European Commission: Brussels, Belgium, 2016; pp. 170–171. [Google Scholar] [CrossRef]
- Pallardy, S. Physiology of Woody Plants. Pac. Conserv. Biol. 1998, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Cavaleri, M.A.; Reed, S.C.; Smith, W.K.; Wood, T.E. Urgent Need for Warming Experiments in Tropical Forests. Glob. Chang. Biol. 2015, 21, 2111–2121. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas Exchange Measurements, What Can They Tell Us about the Underlying Limitations to Photosynthesis? Procedures and Sources of Error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Mao, Z.; Xu, J. Gas Exchange, Chlorophyll and Growth Responses of Betula Platyphylla Seedlings to Elevated CO2 and Nitrogen. Int. J. Biol. 2010, 2, 143–149. [Google Scholar] [CrossRef]
- Liu, M.Z.; Jiang, G.M.; Li, Y.G.; Gao, L.M.; Niu, S.L.; Cui, H.X.; Ding, L. Gas Exchange, Photochemical Efficiency, and Leaf Water Potential in Three Salix Species. Photosynthetica 2003, 41, 393–398. [Google Scholar] [CrossRef]
- Bojarczuk, K.; Karolewski, P.; Oleksyn, J.; Kieliszewska-Rokicka, B.; Zytkowiak, R.; Tjoelker, M.G. Effect of Polluted Soil and Fertilisation on Growth and Physiology of Silver Birch (Betula pendula Roth.) Seedlings. Polish J. Environ. Stud. 2002, 11, 483–492. [Google Scholar]
- Hermle, S.; Vollenweider, P.; Günthardt-Goerg, M.S.; Mcquattie, C.J.; Matyssek, R. Leaf Responsiveness of Populus tremula and Salix viminalis to Soil Contaminated with Heavy Metals and Acidic Rainwater. Tree Physiol. 2007, 27, 1517–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Cui, B.X.; Zhang, X.X.; Li, K.R. The Effects of Petroleum-Contaminated Soil on Photosynthesis of Amorpha fruticosa Seedlings. Int. J. Environ. Sci. Technol. 2016, 13, 2383–2392. [Google Scholar] [CrossRef] [Green Version]
- Horton, J.L.; Kolb, T.E.; Hart, S.C. Leaf Gas Exchange Characteristics Differ among Sonoran Desert Riparian Tree Species. Tree Physiol. 2001, 21, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.F.; Li, X.; Su, Y.H.; Huang, C.L. Parameterization of a Coupled CO2 and H2O Gas Exchange Model at the Leaf Scale of Populus euphratica. Hydrol. Earth Syst. Sci. 2010, 14, 419–431. [Google Scholar] [CrossRef] [Green Version]
- Rood, S.B.; Nielsen, J.L.; Shenton, L.; Gill, K.M.; Letts, M.G. Effects of Flooding on Leaf Development, Transpiration, and Photosynthesis in Narrowleaf Cottonwood, a Willow-like Poplar. Photosynth. Res. 2010, 104, 31–39. [Google Scholar] [CrossRef]
- Andralojc, P.J.; Bencze, S.; Madgwick, P.J.; Philippe, H.; Powers, S.J.; Shield, I.; Karp, A.; Parry, M.A.J. Photosynthesis and Growth in Diverse Willow Genotypes. Food Energy Secur. 2014, 3, 69–85. [Google Scholar] [CrossRef]
- Talbot, R.J.; Etherington, J.R.; Bryant, J.A. Comparative Studies of Plant Growth and Distribution in Relation to Waterlogging. XII. Growth, Photosynthetic Capacity and Metal Ion Uptake in Salix Caprea and S. Cinerea Ssp. Oleifolia. New Phytol. 1987, 105, 563–574. [Google Scholar] [CrossRef]
- Johnson, J.D.; Tognetti, R.; Paris, P. Water Relations and Gas Exchange in Poplar and Willow under Water Stress and Elevated Atmospheric CO2. Physiol. Plant. 2002, 115, 93–100. [Google Scholar] [CrossRef]
- Aalto, T.; Juurola, E. Parametrization of a Biochemical CO2 Exchange Model for Birch (Betula pendula Roth.). Boreal Environ. Res. 2001, 6, 53–64. [Google Scholar]
- Oleksyn, J.; Żytkowiak, R.; Reich, P.B.; Tjoelker, M.G.; Karolewski, P. Ontogenetic Patterns of Leaf CO 2 Exchange, Morphology and Chemistry in Betula Pendula Trees. Trees Struct. Funct. 2000, 14, 271–281. [Google Scholar] [CrossRef]
- Hoshika, Y.; Watanabe, M.; Inada, N.; Mao, Q.; Koike, T. Photosynthetic Response of Early and Late Leaves of White Birch (Betula platyphylla Var. Japonica) Grown under Free-Air Ozone Exposure. Environ. Pollut. 2013, 182, 242–247. [Google Scholar] [CrossRef]
- Côté, B.; Carlson, R.W.; Dawson, J.O. Leaf Photosynthetic Characteristics of Seedlings of Actinorhizal Alnus Spp. and Elaeagnus Spp. Photosynth. Res. 1988, 16, 211–218. [Google Scholar] [CrossRef]
- Piper, F.I.; Cavieres, L.A. Gas Exchange of Juvenile and Mature Trees of Alnus jorullensis (Betulaceae) at Sites with Contrasting Humidity in the Venezuelan Andes. Ecol. Res. 2010, 25, 51–58. [Google Scholar] [CrossRef]
- Popa, A.; Popa, I. Caracteristici ale procesului de fotosinteză la aninul verde din Munții Călimani. Rev. Silvic. Cinegetică 2019, 44, 20–23. [Google Scholar]
- Gorea, D.; Hancu, G.; Rusu, A.; Cârje, A.; Barabás, E. Quality Assessment Indicators of Surface Waters and Soils in the Vicinity of the Former Sulfur Mine in the Călimani Mountains. Forum Geogr. 2014, XIII, 212–218. [Google Scholar] [CrossRef]
- Erzberger, P.; Höhn, M.; Pócs, T. Contribution to the Bryoflora of Călimani Mountains in the Eastern Carpathians, Romania, I. Acta Biol. Plant. Agriensis 2012, 2, 75–97. [Google Scholar]
- LI-COR. Using the LI-6800 Portable Photosynthesis System for Bluestem OSTM Version 1.3; Li-Cor Biosciences: Lincoln, NE, USA, 2018; Volume 1. [Google Scholar]
- Covshoff, S. Photosynthesis Methods and Protocols; Humana Press: Totowa, NJ, USA, 2018; Volume 1770. [Google Scholar] [CrossRef]
- Marozas, V.; Augustaitis, A.; Pivoras, A.; Baumgarten, M.; Mozgeris, G.; Sasnauskienė, J.; Dautartė, A.; Abraitienė, J.; Bičenkienė, S.; Mordas, G.; et al. Comparative Analyses of Gas Exchange Characteristics and Chlorophyll Fluorescence of Three Dominant Tree Species during the Vegetation Season in Hemi-Boreal Zone, Lithuania. J. Agric. Meteorol. 2019, 75, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Terashima, I. Anatomy of Non-Uniform Leaf Photosynthesis. Photosynth. Res. 1992, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 Leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Struik, P.C.; Romero, P.; Harbinson, J.; Evers, J.B.; Van Der Putten, P.E.L.; Vos, J. Using Combined Measurements of Gas Exchange and Chlorophyll Fluorescence to Estimate Parameters of a Biochemical C3 Photosynthesis Model: A Critical Appraisal and a New Integrated Approach Applied to Leaves in a Wheat (Triticum aestivum) Canopy. Plant Cell Environ. 2009, 32, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature Response of Parameters of a Biochemically Based Model of Photosynthesis. II. A Review of Experimental Data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R.; Long, S.P. Improved Temperature Response Functions for Models of Rubisco-Limited Photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Duursma, R.A. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.J.B.; Fiscus, E.L.; Booker, F.L.; Flowers, M.D.; Reid, C.D. Optimizing the Statistical Estimation of the Parameters of the Farquhar-von Caemmerer-Berry Model of Photosynthesis. New Phytol. 2007, 176, 402–414. [Google Scholar] [CrossRef]
- Yin, X.; Struik, P.C. Theoretical Reconsiderations When Estimating the Mesophyll Conductance to CO2 Diffusion in Leaves of C3 Plants by Analysis of Combined Gas Exchange and Chlorophyll Fluorescence Measurements. Plant Cell Environ. 2009, 32, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Moualeu-Ngangue, D.P.; Chen, T.W.; Stützel, H. A New Method to Estimate Photosynthetic Parameters through Net Assimilation Rate−intercellular Space CO2 Concentration (A−Ci) Curve and Chlorophyll Fluorescence Measurements. New Phytol. 2017, 213, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Tukey’s Honestly Significant Difference (HSD) Test. Encycl. Res. Des. 2010, 3, 1–5. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R--project.org (accessed on 2 May 2021).
- Maurer, S.; Matyssek, R. Nutrition and the Ozone Sensitivity of Birch (Betula Pendula) II. Carbon Balance, Water-Use Efficiency and Nutritional Status of the Whole Plant. Trees Struct. Funct. 1997, 12, 11–20. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, T.; Zhang, Y. Effects of Experimental Warming on Phenology, Growth and Gas Exchange of Treeline Birch (Betula utilis) Saplings, Eastern Tibetan Plateau, China. Eur. J. For. Res. 2012, 131, 811–819. [Google Scholar] [CrossRef]
- Hanson, D.T.; Stutz, S.S.; Boyer, J.S. Why Small Fluxes Matter: The Case and Approaches for Improving Measurements of Photosynthesis and (Photo)Respiration. J. Exp. Bot. 2016, 67, 3027–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collatz, G.J.; Ball, J.T.; Grivet, C.; Berry, J.A. Physiological and Environmental Regulation of Stomatal Conductance, Photosynthesis and Transpiration: A Model That Includes a Laminar Boundary Layer. Agric. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Kaluthota, S.; Pearce, D.W.; Evans, L.M.; Letts, M.G.; Whitham, T.G.; Rood, S.B. Higher Photosynthetic Capacity from Higher Latitude: Foliar Characteristics and Gas Exchange of Southern, Central and Northern Populations of Populus angustifolia. Tree Physiol. 2015, 35, 936–948. [Google Scholar] [CrossRef] [Green Version]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From Leaf to Whole-Plant Water Use Efficiency (WUE) in Complex Canopies: Limitations of Leaf WUE as a Selection Target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.J.; Adams, M.A.; Amthor, J.S.; Barbour, M.M.; Berry, J.A.; Ellsworth, D.S.; Farquhar, G.D.; Ghannoum, O.; Lloyd, J.; McDowell, N.; et al. Sensitivity of Plants to Changing Atmospheric CO2 Concentration: From the Geological Past to the next Century. New Phytol. 2013, 197, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Alía, L.A.; Lüttschwager, D.; Ewald, D. Investigation of Gas Exchange and Biometric Parameters in Isogenic Lines of Poplar Differing in Ploidy. Silvae Genet. 2015, 64, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Bühlmann, T.; Hiltbrunner, E.; Körner, C. Alnus Viridis Expansion Contributes to Excess Reactive Nitrogen Release, Reduces Biodiversity and Constrains Forest Succession in the Alps. Alp. Bot. 2014, 124, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Koike, T. Autumn coloring, photosynthetic performance and leaf development of deciduous broad-leaved trees in relation to forest succession. Tree Physiol. 1990, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Manter, D.K.; Kerrigan, J. A/Ci Curve Analysis across a Range of Woody Plant Species: Influence of Regression Analysis Parameters and Mesophyll Conductance. J. Exp. Bot. 2004, 55, 2581–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, T.; Elings, A.; Dieleman, J.A.; Gort, G.; Marcelis, L.F.M. Estimation of Photosynthesis Parameters for a Modified Farquhar-von Caemmerer-Berry Model Using Simultaneous Estimation Method and Nonlinear Mixed Effects Model. Environ. Exp. Bot. 2012, 82, 66–73. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. Models of Photosynthesis. Plant Physiol. 2001, 125, 42–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Z.; Xu, M.; Lathrop, R.G.; Wang, Y. Comparison of the A-Cc Curve Fitting Methods in Determining Maximum Ribulose 1·5-Bisphosphate Carboxylase/Oxygenase Carboxylation Rate, Potential Light Saturated Electron Transport Rate and Leaf Dark Respiration. Plant Cell Environ. 2009, 32, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Rostamza, M.; McNickle, G.G. A Global Database of Photosynthesis Model Parameters, and Phylogenetically Controlled Analysis of Photosynthetic Responses from Every Major Terrestrial Plant Clade. bioRxiv 2020. [Google Scholar] [CrossRef]




| Parameters | Green Alder | Birch | Aspen | Goat Willow |
|---|---|---|---|---|
| A (μmol CO2·m−2·s−1) | 17.35 ± 3.62 a | 16.62 ± 2.47 ab | 14.43 ± 2.93 bc | 13.31 ± 4.97 c |
| E (mmol H2O·m−2·s−1) | 2.6 ± 0.78 a | 3.44 ± 0.94 b | 2.94 ± 1.16 ab | 2.75 ± 1.34 ab |
| gsw (mol H2O·m−2·s−1) | 0.24 ± 0.10 a | 0.31 ± 0.08 a | 0.27 ± 0.10 a | 0.25 ± 0.12 a |
| Ci (µmol·mol−1) | 240.76 ± 26.07 a | 278.21 ± 13.61 b | 272.36 ± 29.13 b | 274.16 ± 24.30 b |
| A/Ci (μmol CO2·m−2·s−1/µmol·mol−1) | 0.07 ± 0.01 a | 0.06 ± 0.01 b | 0.05 ± 0.01 bc | 0.05 ± 0.02 c |
| WUE (μmol CO2·m−2·s−1/mmol H2O·m−2·s−1) | 6.92 ± 1.08 a | 5.07 ± 1.09 b | 5.63 ± 2.07 b | 5.49 ± 1.78 b |
| iWUE (µmol·mol−1) | 78.05 ± 18.91 a | 54.92 ± 8.85 b | 60.93 ± 20.73 b | 60.9 ± 18.41 b |
| Parameters | E | gsw | Ci |
|---|---|---|---|
| Green alder | |||
| A | 0.80 ** | 0.87 ** | 0.73 ** |
| E | 0.88 ** | 0.84 ** | |
| gsw | 0.93 ** | ||
| Birch | |||
| A | 0.74 ** | 0.77 ** | −0.13 |
| E | 0.94 ** | 0.46 * | |
| gsw | 0.52 ** | ||
| Aspen | |||
| A | 0.84 ** | 0.84 ** | 0.66 ** |
| E | 0.96 ** | 0.82 ** | |
| gsw | 0.87 ** | ||
| Goat willow | |||
| A | 0.93 ** | 0.94 ** | 0.45 * |
| E | 0.97 ** | 0.66 ** | |
| gsw | 0.67 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Popa, I. Photosynthesis Traits of Pioneer Broadleaves Species from Tailing Dumps in Călimani Mountains (Eastern Carpathians). Forests 2021, 12, 658. https://doi.org/10.3390/f12060658
Popa A, Popa I. Photosynthesis Traits of Pioneer Broadleaves Species from Tailing Dumps in Călimani Mountains (Eastern Carpathians). Forests. 2021; 12(6):658. https://doi.org/10.3390/f12060658
Chicago/Turabian StylePopa, Andrei, and Ionel Popa. 2021. "Photosynthesis Traits of Pioneer Broadleaves Species from Tailing Dumps in Călimani Mountains (Eastern Carpathians)" Forests 12, no. 6: 658. https://doi.org/10.3390/f12060658