Effects of Elevated CO2 Concentration and Nitrogen Addition on Soil Respiration in a Cd-Contaminated Experimental Forest Microcosm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Measurement of Rs, Soil Temperature, and Soil Moisture
2.4. Sampling and Measurements
2.5. Data Analysis
3. Results
3.1. Soil Properties
3.2. Litterfall and Fine Root Biomass
3.3. Carbon-degrading Enzymes
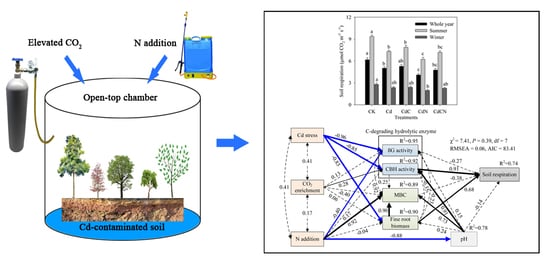
3.4. Soil Respiration
3.5. Factors Controlling Soil Respiration
4. Discussion
4.1. Seasonal Dynamics of Rs
4.2. Effect of Cd Addition on Rs
4.3. Effect of Elevated CO2 on Rs in Cd-contaminated Soil
4.4. Effect of N Addition on Rs in Cd-contaminated Soil
4.5. Effect of Combined Elevated CO2 and N Addition on Rs in Cd-contaminated Soil
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Core Writing Team. Climate Change 2014: Synthesis Report, Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Hu, H.; Xie, N.; Fang, D.; Zhang, X. The role of renewable energy consumption and commercial services trade in carbon dioxide reduction: Evidence from 25 developing countries. Appl. Energy 2018, 211, 1229–1244. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Li, F.; Pei, J.; Ren, J.; Gong, Y.; Yuan, Z.; Ke, W.; Zheng, Y.; Bai, X.; Ye, J. Impacts of warming and nitrogen addition on soil autotrophic and heterotrophic respiration in a semi-arid environment. Agric. For. Meteorol. 2018, 248, 449–457. [Google Scholar] [CrossRef]
- Chen, J.; He, F.; Zhang, X.; Sun, X.; Zheng, J.; Zheng, J. Heavy metal pollution decreases microbial abundance, diversity and activity within particle-size fractions of a paddy soil. FEMS Microbiol. Ecol. 2014, 87, 164–181. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 1992, 44, 81–99. [Google Scholar] [CrossRef] [Green Version]
- King, J.S.; Hanson, P.J.; Bernhardt, E.; DeAngelis, P.; Norby, R.J.; Pregitzer, K.S. A multiyear synthesis of soil respiration responses to elevated atmospheric CO2 from four forest FACE experiments. Glob. Chang. Biol. 2004, 10, 1027–1042. [Google Scholar] [CrossRef]
- Drake, J.E.; Macdonald, C.A.; Tjoelker, M.G.; Reich, P.B.; Singh, B.K.; Anderson, I.C.; Ellsworth, D.S. Three years of soil respiration in a mature eucalypt woodland exposed to atmospheric CO2 enrichment. Biogeochemistry 2018, 139, 85–101. [Google Scholar] [CrossRef]
- Oishi, A.C.; Palmroth, S.; Johnsen, K.H.; McCarthy, H.R.; Oren, R. Sustained effects of atmospheric [CO2] and nitrogen availability on forest soil CO2 efflux. Glob. Chang. Biol. 2014, 20, 1146–1160. [Google Scholar] [CrossRef]
- Lukac, M.; Calfapietra, C.; Godbold, D.L. Production, turnover and mycorrhizal colonization of root systems of three Populus species grown under elevated CO2 (POPFACE). Glob. Chang. Biol. 2003, 9, 838–848. [Google Scholar] [CrossRef]
- Jackson, R.B.; Cook, C.W.; Pippen, J.S.; Palmer, S.M. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 2009, 90, 3352–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, S.G.; Taylor, B.N.; Cooper, E.R.; Beidler, K.V.; Strand, A.E.; McCormack, M.L.; Zhang, S. Long-term dynamics of mycorrhizal root tips in a loblolly pine forest grown with free-air CO2 enrichment and soil N fertilization for 6 years. Glob. Chang. Biol. 2014, 20, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.K.F.; Leuzinger, S.; Keel, S.G.; Siegwolf, R.T.W.; Hagedorn, F.; Schleppi, P.; Körner, C.; Lee, J. Central European hardwood trees in a high-CO2 future: Synthesis of an 8-year forest canopy CO2 enrichment project. J. Ecol. 2013, 101, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Hasselquist, N.J.; Palmroth, S.; Zheng, Z.M.; You, W.H. Short-term response of soil respiration to nitrogen fertilization in a subtropical evergreen forest. Soil Biol. Biochem. 2014, 76, 297–300. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, J.J.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Fu, S.L.; Yan, E.R.; Wu, J.S.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Maaroufi, N.I.; Nordin, A.; Hasselquist, N.J.; Bach, L.H.; Palmqvist, K.; Gundale, M.J. Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Glob. Chang. Biol. 2015, 21, 3169–3180. [Google Scholar] [CrossRef]
- Wang, Q.K.; Zhang, W.D.; Sun, T.; Chen, L.C.; Pang, X.Y.; Wang, Y.P.; Xiao, F.M. N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric. For. Meteorol. 2017, 232, 66–73. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Chang. Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Yan, W.; Shangguan, Z. The effects of nitrogen enrichment on soil CO2 fluxes depending on temperature and soil properties. Glob. Ecol. Biogeogr. 2016, 25, 475–488. [Google Scholar] [CrossRef]
- Vose, J.M.; Elliott, K.J.; Johnson, D.W.; Walker, R.F.; Johnson, M.G.; Tingey, D.T. Effects of elevated CO2 and N fertilization on soil respiration from ponderosa pine (Pinus ponderosa) in open-top chambers. Can. J. For. Res. 1995, 25, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Zhou, G.; Liu, J.; Liu, S.; Duan, H.; Zhang, D. Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 2010, 7, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Sawicka-Kapusta, K.; Zakrzewska, M.; Bajorek, K.; Gdula-Argasinska, J. Input of heavy metals to the forest floor as a result of Cracow urban pollution. Environ. Int. 2003, 28, 691–698. [Google Scholar] [CrossRef]
- Sun, F.F.; Wen, D.Z.; Kuang, Y.W.; Li, J.; Zhang, J.G. Concentrations of sulphur and heavy metals in needles and rooting soils of Masson pine (Pinus massoniana L.) trees growing along an urban-rural gradient in Guangzhou, China. Environ. Monit. Assess. 2009, 154, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Yang, W.; Tan, B.; Peng, Y.; Huang, C.; Xu, Z.; Ni, X.; Yang, Y.; Zhou, W.; Zhang, L.; et al. Immobilization of heavy metals during aquatic and terrestrial litter decomposition in an alpine forest. Chemosphere 2019, 216, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Z.; Li, D.; Nie, X.; Xie, Z.; Chen, J.; Liang, C.; Liao, Y.; Liu, T. Loss characteristics of Cd in soil aggregates under simulated rainfall conditions. Sci. Total Environ. 2019, 650, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Gülser, F.; Erdoğan, E. The effects of heavy metal pollution on enzyme activities and basal soil respiration of roadside soils. Environ. Monit. Assess. 2008, 145, 127–133. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Q.; Liu, Y.J.; Jia, F.A.; He, X.H. Responses of soil microbial activity to cadmium pollution and elevated CO2. Sci. Rep. 2014, 4, 4287. [Google Scholar] [CrossRef]
- Wu, F.; Yang, W.; Zhang, J.; Zhou, L. Cadmium accumulation and growth responses of a poplar (Populus deltoids × Populus nigra) in cadmium contaminated purple soil and alluvial soil. J. Hazard. Mater. 2010, 177, 268–273. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Zhou, G.; Zhang, D.; Liu, S.; Li, Y. Nitrogen to phosphorus ratios of tree species in response to elevated carbon dioxide and nitrogen addition in subtropical forests. Glob. Chang. Biol. 2013, 19, 208–216. [Google Scholar] [CrossRef]
- Li, J.; Fang, Y.T.; Yoh, M.; Wang, X.M.; Wu, Z.Y.; Kuang, Y.W.; Wen, D.Z. Organic nitrogen deposition in precipitation in metropolitan Guangzhou city of southern China. Atmos. Res. 2012, 113, 57–67. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Lin, A.; Ye, Z.; Huang, M. Heavy metal contamination in Pearl River Delta—status and research priorities. Acta Sci. Circumst. 2005, 12, 1575–1579, (In Chinese with English Abstract). [Google Scholar]
- Hagedorn, F.; Hiltbrunner, D.; Streit, K.; Ekblad, A.; Lindahl, B.; Miltner, A.; Frey, B.; Handa, I.T.; Hättenschwiler, S. Nine years of CO2 enrichment at the alpine treeline stimulates soil respiration but does not alter soil microbial communities. Soil Biol. Biochem. 2013, 57, 390–400. [Google Scholar] [CrossRef]
- Huang, S.; Jia, X.; Zhao, Y.; Bai, B.; Chang, Y. Elevated CO2 benefits the soil microenvironment in the rhizosphere of Robinia pseudoacacia L. seedlings in Cd- and Pb-contaminated soils. Chemosphere 2017, 168, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.K.; Mori, T.; Mao, Q.G.; Zhou, K.J.; Zhou, G.Y.; Nie, Y.X.; Mo, J.M. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. Jove-J. Vis. Exp. 2013, 81, e50961. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.L.; Zhou, G.Y.; Liu, J.X.; Liu, S.Z.; Zhang, Q.F.; Zhang, D.Q. Seasonal responses of soil respiration to elevated CO2 and N addition in young subtropical forest ecosystems in southern China. Ecol. Eng. 2013, 61, 65–73. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Liu, Y.; He, N.P.; Wen, X.F.; Xu, L.; Sun, X.M.; Yu, G.R.; Liang, L.Y.; Schipper, L.A. The optimum temperature of soil microbial respiration: Patterns and controls. Soil Biol. Biochem. 2018, 121, 35–42. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.E.I.; Zhu, W.; Gundersen, P.E.R.; Fang, Y.; Li, D.; Wang, H.U.I. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Khomik, M.; Arain, M.A.; McCaughey, J.H. Temporal and spatial variability of soil respiration in a boreal mixedwood forest. Agric. For. Meteorol. 2006, 140, 244–256. [Google Scholar] [CrossRef]
- Yan, T.; Qu, T.T.; Sun, Z.Z.; Dybzinski, R.; Chen, A.P.; Yao, X.C.; Zeng, H.; Piao, S.L. Negative effect of nitrogen addition on soil respiration dependent on stand age: Evidence from a 7-year field study of larch plantations in northern China. Agric. For. Meteorol. 2018, 262, 24–33. [Google Scholar] [CrossRef]
- Doff sotta, E.; Meir, P.; Malhi, Y.; Donato nobre, A.; Hodnett, M.; Grace, J. Soil CO2 efflux in a tropical forest in the central Amazon. Glob. Chang. Biol. 2004, 10, 601–617. [Google Scholar] [CrossRef]
- Doelman, P.; Haanstra, L. Short-term and long-term effects of cadmium, chromium, copper, nickel, lead and zinc on soil microbial respiration in relation to abiotic soil factors. Plant Soil 1984, 79, 317–327. [Google Scholar] [CrossRef]
- Verma, R.K.; Yadav, D.V.; Singh, C.P.; Suman, A.; Gaur, A. Effect of heavy metals on soil respiration during decomposition of sugarcane (Saccharum officinarum L.) trash in different soils. Plant Soil Environ. 2010, 56, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Epelde, L.; Garbisu, C.; Bernal, M.P. Evaluation of the phytostabilisation efficiency in a trace elements contaminated soil using soil health indicators. J. Hazard. Mater. 2014, 268, 68–76. [Google Scholar] [CrossRef]
- Tyler, G.; Pahlsson, A.M.B.; Bengtsson, G.; Baath, E.; Tranvik, L. Heavy-metal ecology of terrestrial plants, microorganisms and invertebrates—A review. Water Air Soil Pollut. 1989, 47, 189–215. [Google Scholar] [CrossRef]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Brookes, P.C.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Dar, G.H. Effects of cadmium and sewage-sludge on soil microbial biomass and enzyme activities. Bioresour. Technol. 1996, 56, 141–145. [Google Scholar] [CrossRef]
- Moreno, J.L.; Hernandez, T.; Garcia, C. Effects of a cadmium-contaminated sewage sludge compost on dynamics of organic matter and microbial activity in an arid soil. Biol. Fertil. Soils 1999, 28, 230–237. [Google Scholar] [CrossRef]
- Drake, J.E.; Gallet-Budynek, A.; Hofmockel, K.S.; Bernhardt, E.S.; Billings, S.A.; Jackson, R.B.; Johnsen, K.S.; Lichter, J.; McCarthy, H.R.; McCormack, M.L.; et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 2011, 14, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, X.; Shao, J.; Nie, Y.; He, Y.; Jiang, L.; Wu, Z.; Hosseini Bai, S. Interactive effects of global change factors on soil respiration and its components: A meta-analysis. Glob. Chang. Biol. 2016, 22, 3157–3169. [Google Scholar] [CrossRef] [PubMed]
- Palmroth, S.; Oren, R.; McCarthy, H.R.; Johnsen, K.H.; Finzi, A.C.; Butnor, J.R.; Ryan, M.G.; Schlesinger, W.H. Aboveground sink strength in forests controls the allocation of carbon below ground and its [CO2]-induced enhancement. Proc. Natl. Acad. Sci. USA 2006, 103, 19362–19367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.H.; Dai, S.X.; Wang, R.G.; Guo, J.K.; Ding, Y.Z.; Xu, Y.M. Combined effects of elevated CO2 and Cd-contaminated soil on the growth, gas exchange, antioxidant defense, and Cd accumulation of poplars and willows. Environ. Exp. Bot. 2015, 115, 1–10. [Google Scholar] [CrossRef]
- Jackson, R.B.; Sala, O.E.; Field, C.B.; Mooney, H.A. CO2 alters water-use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia 1994, 98, 257–262. [Google Scholar] [CrossRef]
- Nelson, J.A.; Morgan, J.A.; LeCain, D.R.; Mosier, A.R.; Milchunas, D.G.; Parton, B.A. Elevated CO2 increases soil moisture and enhances plant water relations in a long-term field study in semi-arid shortgrass steppe of Colorado. Plant Soil 2004, 259, 169–179. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Gu, H.; Gao, F. Nitrogen deposition and management practices increase soil microbial biomass carbon but decrease diversity in Moso bamboo plantations. Sci. Rep. 2016, 6, 28235. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.B.; Wu, J.P.; Liu, W.F.; Yuan, Y.H.; Huang, R.Z.; Liao, Y.C.; Li, Y.Y. Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant Soil 2014, 379, 361–371. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Northern Temperate Forest Ecosystems. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Tian, D.H.; Wang, H.; Sun, J.; Niu, S.L. Global evidence on nitrogen saturation of terrestrial ecosystem net primary productivity. Environ. Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Song, X.Z.; Chang, S.X.; Peng, C.H.; Xiao, W.F.; Zhang, J.B.; Xiang, W.H.; Li, Y.; Wang, W.F. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.M.; Li, J.J.; Lan, Z.C.; Hu, S.J.; Bai, Y.F. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Su, Y.G.; Huang, G.; Lin, Y.J.; Zhang, Y.M. No synergistic effects of water and nitrogen addition on soil microbial communities and soil respiration in a temperate desert. Catena 2016, 142, 126–133. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytol. 2007, 176, 655–664. [Google Scholar] [CrossRef]
- Norby, R.J.; Jackson, R.B. Root dynamics and global change: Seeking an ecosystem perspective. New Phytol. 2000, 147, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.C.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J.; et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience 2004, 54, 731–739. [Google Scholar] [CrossRef] [Green Version]
- De Graaff, M.A.; van Groenigen, K.J.; Six, J.; Hungate, B.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Butnor, J.R.; Johnsen, K.H.; Oren, R.; Katul, G.G. Reduction of forest floor respiration by fertilization on both carbon dioxide-enriched and reference 17-year-old loblolly pine stands. Glob. Chang. Biol. 2003, 9, 849–861. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F.; Paul, K.I. Modelling C and N dynamics in forest soils with a modified version of the CENTURY model. Soil Biol. Biochem. 2002, 34, 341–354. [Google Scholar] [CrossRef]
- Miehle, P.; Livesley, S.J.; Feikema, P.M.; Li, C.; Arndt, S.K. Assessing productivity and carbon sequestration capacity of Eucalyptus globulus plantations using the process model forest-DNDC: Calibration and validation. Ecol. Model. 2006, 192, 83–94. [Google Scholar] [CrossRef]
- Thornton, P.E.; Law, B.E.; Gholz, H.L.; Clark, K.L.; Falge, E.; Ellsworth, D.S.; Golstein, A.H.; Monson, R.K.; Hollinger, D.; Falk, M.; et al. Modeling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agric. For. Meteorol. 2002, 113, 185–222. [Google Scholar] [CrossRef]







| Treatments | pH | SOC (mg g−1) | TN (mg g−1) | TP (mg g−1) | TCd (mg kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | Coarse Sand Content (%) | Fine Sand Content (%) | Silt and Clay Content (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| CK | 5.44 ± 0.13 | 2.24 ± 0.12 | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.96 ± 0.17 | 2.23 ± 0.17 | 1.03 ± 0.31 | 50.8 ± 1.1 | 21.5 ± 0.0 | 27.7 ± 1.1 |
| Cd | 5.26 ± 0.03 | 2.37 ± 0.27 | 0.19 ± 0.02 | 0.12 ± 0.00 | 0.97 ± 0.16 | 2.45 ± 0.23 | 0.99 ± 0.26 | 54.6 ± 0.8 | 18.5 ± 0.3 | 26.9 ± 0.6 |
| CdC | 5.23 ± 0.01 | 2.34 ± 0.08 | 0.20 ± 0.01 | 0.12 ± 0.01 | 0.91 ± 0.05 | 2.30 ± 0.18 | 1.06 ± 0.28 | 53.7 ± 0.2 | 19.8 ± 0.1 | 26.5 ± 0.1 |
| CdN | 5.34 ± 0.05 | 2.63 ± 0.18 | 0.18 ± 0.01 | 0.12 ± 0.01 | 0.97 ± 0.13 | 2.53 ± 0.12 | 0.99 ± 0.32 | 52.0 ± 2.2 | 19.1 ± 0.7 | 28.8 ± 1.6 |
| CdCN | 5.25 ± 0.08 | 2.50 ± 0.33 | 0.16 ± 0.03 | 0.13 ± 0.01 | 0.94 ± 0.06 | 2.31 ± 0.34 | 1.09 ± 0.15 | 50.1 ± 0.6 | 21.7 ± 0.4 | 28.2 ± 0.2 |
| Variable | Season | Treatments | ||||
|---|---|---|---|---|---|---|
| CK | Cd | CdC | CdN | CdCN | ||
| pH | summer | 5.46 ± 0.24a | 5.33 ± 0.11a | 5.36 ± 0.11a | 4.68 ± 0.08b | 4.64 ± 0.02b |
| winter | 5.37 ± 0.00a | 5.31 ± 0.04a | 5.25 ± 0.01a | 4.66 ± 0.04b | 4.66 ± 0.07b | |
| SOC (mg g−1) | summer | 4.08 ± 0.48a | 4.33 ± 0.54a | 3.71 ± 0.11a | 3.53 ± 0.28a | 3.75 ± 0.25a |
| winter | 3.19 ± 0.24a | 3.63 ± 0.18a | 3.94 ± 0.36a | 3.33 ± 0.08a | 3.31 ± 0.25a | |
| TN (mg g−1) | summer | 0.28 ± 0.03a | 0.34 ± 0.01a | 0.31 ± 0.00a | 0.28 ± 0.04a | 0.29 ± 0.01a |
| winter | 0.24 ± 0.02ab | 0.23 ± 0.00b | 0.26 ± 0.01ab | 0.26 ± 0.00ab | 0.29 ± 0.02a | |
| C:N ratio | summer | 14.85 ± 2.15a | 12.65 ± 1.32a | 12.07 ± 0.31a | 12.69 ± 1.35a | 12.80 ± 0.40a |
| winter | 13.47 ± 0.46ab | 16.01 ± 0.84a | 15.23 ± 1.93a | 12.67 ± 0.19ab | 11.62 ± 0.51b | |
| TCd (mg kg−1) | summer | 1.02 ± 0.12d | 85.39 ± 6.40a | 70.10 ± 5.80b | 21.22 ± 5.77c | 20.49 ± 1.62c |
| winter | 0.57 ± 0.03d | 87.43 ± 6.35a | 75.34 ± 1.98b | 24.24 ± 1.05c | 25.00 ± 3.03c | |
| MBC (mg kg−1) | summer | 84.80 ± 11.05a | 52.06 ± 1.92b | 52.80 ± 3.92b | 48.73 ± 7.98b | 38.43 ± 2.25b |
| winter | 80.78 ± 9.09a | 40.09 ± 6.99b | 48.01 ± 6.66b | 44.98 ± 12.19b | 52.66 ± 2.46b | |
| NH4+-N (mg kg−1) | summer | 5.90 ± 0.55b | 5.76 ± 0.44b | 5.61 ± 0.54b | 10.56 ± 1.62a | 10.94 ± 1.24a |
| winter | 3.60 ± 0.73b | 5.79 ± 0.78b | 6.61 ± 0.22b | 11.97 ± 1.49a | 14.63 ± 3.26a | |
| NO3−-N (mg kg−1) | summer | 0.86 ± 0.34b | 0.98 ± 0.23b | 1.68 ± 0.47b | 6.51 ± 1.41a | 7.12 ± 1.29a |
| winter | 3.07 ± 0.41b | 4.15 ± 0.64b | 5.69 ± 1.29b | 10.52 ± 1.08a | 12.67 ± 0.98a | |
| Treatment | Rs = a expbT | Rs = k M + c | ||||
|---|---|---|---|---|---|---|
| a | b | R2 | k | c | R2 | |
| CK | 0.6283 | 0.0917 | 0.72 ** | 0.2072 | 2.2626 | 0.11 * |
| Cd | 0.5941 | 0.0863 | 0.75 ** | 0.2211 | 0.8834 | 0.17 ** |
| CdC | 0.5921 | 0.0891 | 0.77 ** | 0.1948 | 1.7273 | 0.13 * |
| CdN | 0.5159 | 0.0842 | 0.74 ** | 0.1849 | 0.3509 | 0.16 ** |
| CdCN | 0.5439 | 0.0885 | 0.79 ** | 0.2050 | 0.6976 | 0.21 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, B.; Hu, Q.; Zhang, G.; Yi, Y.; Xiao, M.; Wen, D. Effects of Elevated CO2 Concentration and Nitrogen Addition on Soil Respiration in a Cd-Contaminated Experimental Forest Microcosm. Forests 2020, 11, 260. https://doi.org/10.3390/f11030260
Yao B, Hu Q, Zhang G, Yi Y, Xiao M, Wen D. Effects of Elevated CO2 Concentration and Nitrogen Addition on Soil Respiration in a Cd-Contaminated Experimental Forest Microcosm. Forests. 2020; 11(3):260. https://doi.org/10.3390/f11030260
Chicago/Turabian StyleYao, Bo, Qiwu Hu, Guihua Zhang, Yafeng Yi, Meijuan Xiao, and Dazhi Wen. 2020. "Effects of Elevated CO2 Concentration and Nitrogen Addition on Soil Respiration in a Cd-Contaminated Experimental Forest Microcosm" Forests 11, no. 3: 260. https://doi.org/10.3390/f11030260