Changes in Proline Levels during Seed Development of Orthodox and Recalcitrant Seeds of Genus Acer in a Climate Change Scenario
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials Collection
2.2. Proline Extraction and Determination
2.3. Water Content Determination
2.4. Meteorology Data
2.5. Statistical Analysis
3. Results and Discussion
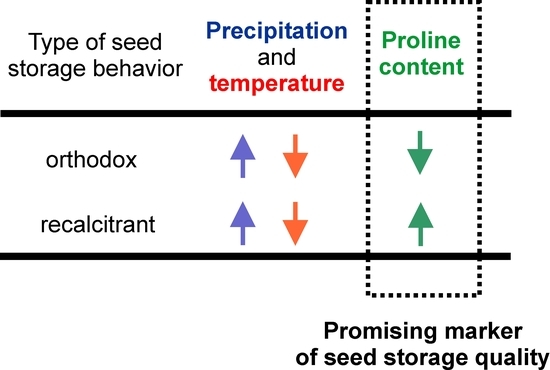
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.-Q.; Liu, S.-J.; Song, S.-Q.; Møller, I.M. Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol. Biochem. 2015, 86, 1–15. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Nasser, A.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Berjak, P.; Pammenter, N.W. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef] [Green Version]
- Pukacka, S.; Wójkiewicz, E. Carbohydrate metabolism in Norway maple and sycamore seeds in relation to desiccation tolerance. J. Plant Physiol. 2002, 159, 273–279. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Ascorbate and glutathione metabolism during development and desiccation of orthodox and recalcitrant seeds of the genus Acer. Funct. Plant Biol. 2007, 34, 601–613. [Google Scholar] [CrossRef]
- Pukacka, S.; Hoffmann, S.K.; Goslar, J.; Pukacki, P.M.; Wójkiewicz, E. Water and lipid relations in beech (Fagus sylvatica L.) seeds and its effect on storage behaviour. Biochim. Biophys. Acta Bioenerg. 2003, 1621, 48–56. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Age-related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Sci. Res. 2007, 17, 45–53. [Google Scholar] [CrossRef]
- Leprince, O.; Hendry, G.A.F.; McKersie, B.D. The mechanisms of desiccation tolerance in developing seeds. Seed Sci. Res. 1993, 3, 231–246. [Google Scholar] [CrossRef]
- Staszak, A.M.; Pawłowski, T.A. Proteomic Analysis of Embryogenesis and the Acquisition of Seed Dormancy in Norway Maple (Acer platanoides L.). Int. J. Mol. Sci. 2014, 15, 10868–10891. [Google Scholar] [CrossRef] [Green Version]
- Berjak, P.; Farrant, J.M.; Pammenter, N.W. Seed Desiccation-Tolerance Mechanisms in Plant Desiccation Tolerance, 1st ed.; Jenks, M.A., Wood, A.J., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 151–192. ISBN 978-0-8138-1263-2. [Google Scholar]
- Leprince, O.; Buitink, J.; Hoekstra, F.A. Axes and cotyledons of recalcitrant seeds of Castanea sativa Mill. exhibit contrasting responses of respiration to drying in relation to desiccation sensitivity. J. Exp. Bot. 1999, 50, 1515–1524. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Ashraf, M.; Shahbaz, M.; Humera, H. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 2008, 40, 211–219. [Google Scholar]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Shevyakova, N.I.; Bakulina, E.A.; Kuznetsov, V.V. Proline antioxidant role in the common ice plant subjected to salinity and paraquat treatment inducing oxidative stress. Russ. J. Plant Physiol. 2009, 56, 663–669. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, S.; Thakur, P.; Malik, J.A.; Bhandhari, K.; Sharma, K.D.; Nayyar, H. Involvement of proline in response of chickpea (Cicer arietinum L.) to chilling stress at reproductive stage. Sci. Hortic. 2011, 128, 174–181. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Hossain, M.; Kishor, P. Engineering Proline Metabolism for Enhanced Plant Salt Stress Tolerance. Manag. Salt Toler. Plants 2015, 353–372. [Google Scholar]
- Schertl, P.; Cabassa, C.; Saadallah, K.; Bordenave, M.; Savouré, A.; Braun, H.-P. Biochemical characterization of proline dehydrogenase in Arabidopsis mitochondria. FEBS J. 2014, 281, 2794–2804. [Google Scholar] [CrossRef] [Green Version]
- Margutti, M.P.; Reyna, M.; Meringer, M.V.; Racagni, G.E.; Villasuso, A.L. Lipid signalling mediated by PLD/PA modulates proline and H2O2 levels in barley seedlings exposed to short- and long-term chilling stress. Plant Physiol. Biochem. 2017, 113, 149–160. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef]
- Ratajczak, E.; Dietz, K.-J.; Kalemba, E.M. The Occurrence of Peroxiredoxins and Changes in Redox State in Acer platanoides and Acer pseudoplatanus During Seed Development. J. Plant Growth Regul. 2018, 38, 298–314. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, M.F.; Donald, G.; Reid, D.M.; Yeung, E.C.; Stasolla, C. Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). J. Exp. Bot. 2005, 56, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Tobias, M.N.; Pammenter, N.W. Dehydration Kinetics of Embryonic Axes from Desiccation-sensitive Seeds: An Assessment of Descriptive Models. J. Integr. Plant Biol. 2009, 51, 1002–1007. [Google Scholar] [CrossRef]
- Dickie, J.B.; May, K.; Morris, S.V.A.; Titley, S.E. The effects of desiccation on seed survival in Acer platanoides L. and Acer pseudoplatanus L. Seed Sci. Res. 1991, 1, 149–162. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Hell, R. Thiol switches in redox regulation of chloroplasts: Balancing redox state, metabolism and oxidative stress. Biol. Chem. 2015, 396, 483–494. [Google Scholar] [CrossRef] [Green Version]
- De Gara, L.; De Pinto, M.C.; Moliterni, V.M.C.; D’Egidio, M.G. Redox regulation and storage processes during maturation in kernels of Triticum durum. J. Exp. Bot. 2003, 54, 249–258. [Google Scholar] [CrossRef]
- Fu, Y.-B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing underex situseed conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef] [Green Version]
- Bogdziewicz, M.; Kelly, D.; Thomas, P.A.; Lageard, J.G.A.; Hacket-Pain, A. Climate warming disrupts mast seeding and its fitness benefits in European beech. Nat. Plants 2020, 6, 88–94. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Nabity, P.D. A global test for phylogenetic signal in shifts in flowering time under climate change. J. Ecol. 2016, 105, 627–633. [Google Scholar] [CrossRef]
- Dobrowolska, D. Vitality of European Beech (Fagus sylvaticaL.) at the Limit of Its Natural Range in Poland. Pol. J. Ecol. 2015, 63, 260–272. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Fernández-Martínez, M.; Bonal, R.; Belmonte, J.; Espelta, J.M. The Moran Effect and Environmental Vetoes: Phenological Synchrony and Drought Drive Seed Production in a Mediterranean Oak. Proc. R. Soc. B 2017, 284, 20171784. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-Q.; Cheng, H.-Y.; Møller, I.M.; Song, S.-Q. The role of recovery of mitochondrial structure and function in desiccation tolerance of pea seeds. Physiol. Plant. 2011, 144, 20–34. [Google Scholar] [CrossRef]
- Probert, R.; Adams, J.; Coneybeer, J.; Crawford, A.; Hay, F. Seed quality for conservation is critically affected by pre-storage factors. Aust. J. Bot. 2007, 55, 326–335. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. Available online: http://prometheuswiki.publish.csiro.au/tikiindex.php?page=PROTOCOL%3A+Extraction+and+determination+of+proline (accessed on 11 August 2020).
- Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 11 August 2018).
- Makowski, D.; Ben-Shachar, M.S.; Patil, I.; Lüdecke, D. Methods and Algorithms for Correlation Analysis in R. J. Open Source Softw. 2020, 5, 2306. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Pukacka, S. Association of Protective Proteins with Dehydration and Desiccation of Orthodox and Recalcitrant Category Seeds of Three Acer Genus Species. J. Plant Growth Regul. 2011, 31, 351–362. [Google Scholar] [CrossRef]
- Pukacka, S.; Pukacki, P.M. Changes in soluble sugars in relation to desiccation tolerance and effects of dehydration on freezing characteristics of Acer platanoides and Acer pseudoplatanus seeds. Acta Physiol. Plant. 1997, 19, 147–154. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Recalcitrance is not an all-or-nothing situation. Seed Sci. Res. 1994, 4, 263–264. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Aggarwal, M.; Sharma, S.; Kaur, N.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Kaur, R.; Singh, K.; Srivastava, A.; Nayyar, H. Exogenous Proline Application Reduces Phytotoxic Effects of Selenium by Minimising Oxidative stress and Improves Growth in Bean (Phaseolus vulgaris L.) Seedlings. Biol. Trace Elem. Res. 2011, 140, 354–367. [Google Scholar] [CrossRef]
- Hoque, A.; Banu, M.N.A.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.M.; Mundree, S.G.; Williams, B.; Bewley, J.D. Desiccation Tolerance: Avoiding Cellular Damage During Drying and Rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, D.F.; O’Connor, B. Proline specific peptidases. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1997, 1343, 160–186. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Pinfield, N.J.; Stutchbury, P.A.; Bazaid, S.A.; Gwarazimba, V.E.E. Abscisic acid and the regulation of embryo dormancy in the genus Acer. Tree Physiol. 1990, 6, 79–85. [Google Scholar] [CrossRef]
- Schroeder, D.F.; Fernando, V.C.D. Role of ABA in Arabidopsis Salt, Drought, and Desiccation Tolerance. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; InTech: London, UK, 2016; ISBN 978-953-51-2250-0. [Google Scholar]
- Ratajczak, E.; Małecka, A.; Ciereszko, I.; Staszak, A.M. Mitochondria Are Important Determinants of the Aging of Seeds. Int. J. Mol. Sci. 2019, 20, 1568. [Google Scholar] [CrossRef] [Green Version]







| Year | Flowering Date | |
|---|---|---|
| Norway Maple | Sycamore Maple | |
| 2017 | 24 April | 1 May |
| 2018 | 15 April | 22 April |
| 2019 | 5 April | 12 April |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kijowska-Oberc, J.; Staszak, A.M.; Wawrzyniak, M.K.; Ratajczak, E. Changes in Proline Levels during Seed Development of Orthodox and Recalcitrant Seeds of Genus Acer in a Climate Change Scenario. Forests 2020, 11, 1362. https://doi.org/10.3390/f11121362
Kijowska-Oberc J, Staszak AM, Wawrzyniak MK, Ratajczak E. Changes in Proline Levels during Seed Development of Orthodox and Recalcitrant Seeds of Genus Acer in a Climate Change Scenario. Forests. 2020; 11(12):1362. https://doi.org/10.3390/f11121362
Chicago/Turabian StyleKijowska-Oberc, Joanna, Aleksandra M. Staszak, Mikołaj K. Wawrzyniak, and Ewelina Ratajczak. 2020. "Changes in Proline Levels during Seed Development of Orthodox and Recalcitrant Seeds of Genus Acer in a Climate Change Scenario" Forests 11, no. 12: 1362. https://doi.org/10.3390/f11121362