Fast Degradation of Azo Dyes by In Situ Mg-Zn-Ca-Sr Metallic Glass Matrix Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Material Characterization
2.3. Azo Dye Degradation Test
3. Results
3.1. Microstructure
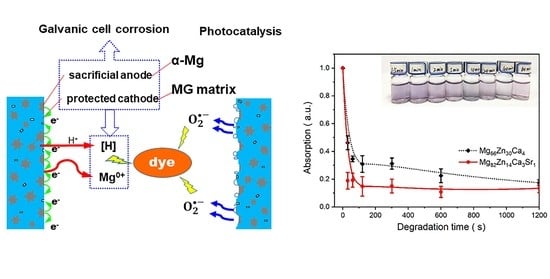
3.2. DB6 Degradation
4. Discussion
5. Conclusions
- (1)
- In situ Mg-Zn-Ca-Sr metallic glass matrix composites with high magnesium content were successfully prepared by melt spinning. SEM, DSC and XRD analyses confirm that the composite is composed of primary α-Mg dendrites, a metallic glass matrix and a few Mg-Zn intermetallic particles.
- (2)
- In-situ Mg-Zn-Ca-Sr MGMCs with different Mg content have similar thermal stability. With the increase in Mg content, the glass transition temperature Tg and the melting point are increased, but the onset crystallization temperature Tx is decreased, resulting in the reduced supercooled liquid region. It indicates that the thermal stability and glass-forming ability of the MG matrix decrease.
- (3)
- The Mg82Zn14Ca3Sr1 MGMC sample with a particle size of up to 500 μm shows a high azo dye degradation rate, indicating that the intrinsic azo dye degradation performance is improved by turning the Mg content. It is sensitive to pH during the treatment of azo dye wastewater, and the acidic environment is conducive to a high degradation rate.
- (4)
- The rapid degradation of azo dyes can be attributed to the sacrificial anode protection induced by a crystalline Mg dendrite, which improves the utilization rate of zero-valent Mg in the MG matrix phase. It is suggested that the azo dye degradation performance of MgZn-based MGMC can be improved by regulating the microstructure of MGMC, which provides a new basis for the design of new dye wastewater treatment materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J. Chemical Functional Applications and Fundamental Researches of Metallic Glasses. Mater. China 2014, 33, 270–281. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Jia, Z.; Lyu, F.; Liang, S.-X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Vandevivere, P.C.; Bianchi, R.; Verstraete, W. Review: Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J. Chem. Technol. Biot. 1998, 72, 289–302. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Excellent capability in degrading azo dyes by MgZn-based metallic glass powders. Sci. Rep. 2012, 2, 418. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-Y.; Ma, E.; Xu, J. Reliability of compressive fracture strength of Mg–Zn–Ca bulk metallic glasses: Flaw sensitivity and Weibull statistics. Scr. Mater. 2008, 58, 496–499. [Google Scholar] [CrossRef]
- Guo, F.; Joseph Poon, S.; Gu, X.; Shiflet, G.J. Low-density Mg-rich metallic glasses with bending ductility. Scr. Mater. 2007, 56, 689–692. [Google Scholar] [CrossRef]
- Yin, H.L.; Yang, W.; Zhao, L.C.; Hu, X.M.; Liu, S.Q.; Cui, C.X.; Wang, X. Fabrication and mechanical property of three-dimensional carbon fiber reinforced Mg-based bulk metallic glass matrix composite. Mater. Sci. Eng. A 2022, 839, 142853. [Google Scholar] [CrossRef]
- Yin, H.L.; Liu, S.Q.; Zhao, L.C.; Cui, C.X.; Wang, X. Vacuum infiltration molding and mechanical property of short carbon fiber reinforced Ti-based metallic glass matrix composite. J. Mater. Process. Tech. 2021, 295, 117151. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Hu, X.; Cheng, Y.; Liu, S.; Chen, P.; Cui, C. Fabrication and Mechanical Behavior of Ex Situ Mg-Based Bulk Metallic Glass Matrix Composite Reinforced with Electroless Cu-Coated SiC Particles. Materials 2017, 10, 1371. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.-h.; Lin, C.-h. Effect of particle size of titanium dioxide nanoparticle aggregates on the degradation of one azo dye. Environ. Sci. Pollut. Res. 2012, 19, 1652–1658. [Google Scholar] [CrossRef]
- Duarte, F.; Maldonado-Hodar, F.J.; Madeira, L.M. Influence of the Particle Size of Activated Carbons on Their Performance as Fe Supports for Developing Fenton-like Catalysts. Ind. Eng. Chem. Res. 2012, 51, 9218–9226. [Google Scholar] [CrossRef]
- Becker, J.; Raghupathi, K.R.; St Pierre, J.; Zhao, D.; Koodali, R.T. Tuning of the Crystallite and Particle Sizes of ZnO Nanocrystalline Materials in Solvothermal Synthesis and Their Photocatalytic Activity for Dye Degradation. J. Phys. Chem. C 2011, 115, 13844–13850. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Si, J.J.; Song, J.G.; Yang, Q.; Hui, X.D. Synthesis of Mg-Zn-Ca metallic glasses by gas-atomization and their excellent capability in degrading azo dyes. Mater. Sci. Eng. B-Adv. 2014, 181, 46–55. [Google Scholar] [CrossRef]
- Iqbal, M.; Wang, W.H. Synthesis and characterization of Mg-based amorphous alloys and their use for decolorization of Azo dyes. In Proceedings of the 13th International Symposium on Advanced Materials (ISAM), Islamabad, Pakistan, 23–27 September 2014. [Google Scholar]
- Wang, X. Surface Crystallization in Mg-Based Bulk Metallic Glass during Copper Mold Casting. Adv. Mater. Sci. Eng. 2014, 2014, 798479. [Google Scholar] [CrossRef] [Green Version]
- Ramya, M.; Karthika, M.; Selvakumar, R.; Raj, B.; Ravi, K.R. A facile and efficient single step ball milling process for synthesis of partially amorphous Mg-Zn-Ca alloy powders for dye degradation. J. Alloys Compd. 2017, 696, 185–192. [Google Scholar] [CrossRef]
- Chen, Q.; Pang, J.; Yan, Z.C.; Hu, Y.H.; Guo, L.Y.; Zhang, H.; Zhang, L.C.; Wang, W.M. MgZn-based amorphous ribbon as a benign decolorizer in methyl blue solution. J. Non-Cryst. Solids 2020, 529, 119802. [Google Scholar] [CrossRef]
- Luo, X.; Li, R.; Zong, J.; Zhang, Y.; Li, H.; Zhang, T. Enhanced degradation of azo dye by nanoporous-copper-decorated Mg-Cu-Y metallic glass powder through dealloying pretreatment. Appl. Surf. Sci. 2014, 305, 314–320. [Google Scholar] [CrossRef]
- Chen, P.; Hu, X.; Qi, Y.; Wang, X.; Li, Z.; Zhao, L.; Liu, S.; Cui, C. Rapid Degradation of Azo Dyes by Melt-Spun Mg-Zn-Ca Metallic Glass in Artificial Seawater. Metals 2017, 7, 485. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yan, Z.; Guo, L.; Zhang, H.; Zhang, L.; Kim, K.; Li, X.; Wang, W. Enhancing the acid orange dye degradation efficiency of Mg-based glassy alloys with introducing porous structure and zinc oxide. J. Alloys Compd. 2020, 831, 154817. [Google Scholar] [CrossRef]
- Xia, Q.; He, S.-Y.; Zhang, W.; Xiang, Q.-C.; Qu, Y.-D.; Ren, Y.-L.; Qiu, K.-Q. Degradation efficiency of Mg65Cu25-xAgxY10 nanoporous dealloyed ribbons on pesticide wastewater. Trans. Nonferrous Met. Soc. 2022, 32, 1472–1484. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H. Mg-based amorphous alloys for decolorization of azo dyes. Results Phys. 2017, 7, 2054–2056. [Google Scholar] [CrossRef]
- Gu, X.; Shiflet, G.J.; Guo, F.Q.; Poon, S.J. Mg–Ca–Zn Bulk Metallic Glasses with High Strength and Significant Ductility. J. Mater. Res. 2005, 20, 1935–1938. [Google Scholar] [CrossRef]
- Ren, Y.; Yue, C.; Wang, T.; Qiu, K.; Li, R. Degrading ability and recycle use characteristics of Mg-Zn-Ca amorphous ribbons in azo dye solution treatment. Trans. Nonferrous Met. Soc. 2016, 26, 2152–2159. [Google Scholar]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg–Zn–Ca–Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Gao, J.; Sharp, J.; Guan, D.; Rainforth, W.M.; Todd, I. New compositional design for creating tough metallic glass composites with excellent work hardening. Acta Mater. 2015, 86, 208–215. [Google Scholar] [CrossRef]
- Lee, J.I.; Ryu, W.H.; Yoon, K.N.; Park, E.S. In-situ synthesis of Mg-based bulk metallic glass matrix composites with primary α-Mg phases. J. Alloys Compd. 2021, 879, 160417. [Google Scholar] [CrossRef]
- Yan, K.; Bai, J.; Liu, H.; Jin, Z.-Y. The precipitation behavior of MgZn2 and Mg4Zn7 phase in Mg-6Zn (wt.%) alloy during equal-channel angular pressing. J. Magnes. Alloy. 2017, 5, 336–339. [Google Scholar] [CrossRef]
- Xie, Y.-P.; Wang, Z.-Y.; Hou, Z.F. The phase stability and elastic properties of MgZn2 and Mg4Zn7 in Mg–Zn alloys. Scr. Mater. 2013, 68, 495–498. [Google Scholar] [CrossRef]
- Lin, X.-p.; Kuo, Y.; Wang, L.; Ye, J.; Zhang, C.; Wang, L.; Guo, K.-y. Refinement and strengthening mechanism of Mg−Zn−Cu−Zr−Ca alloy solidified under extremely high pressure. Trans. Nonferrous Met. Soc. 2021, 31, 1587–1598. [Google Scholar] [CrossRef]
- Luo, Z.P.; Zhang, S.Q.; Tang, Y.L.; Zhao, D.S. On the stable quasicrystals in slowly cooled Mg-Zn-Y alloys. Scr. Metall. Mater. 1995, 32, 1411–1416. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, X.; Wang, X.; Zhang, M.; Hu, H.; Gong, P.; Wang, X. Preparation and mechanical properties of tungsten-particle-reinforced Zr-based bulk-metallic-glass composites. Mater. Sci. Eng. A 2021, 815, 141312. [Google Scholar] [CrossRef]
- Wang, X.; Gong, P.; Yao, K.-F. Mechanical behavior of bulk metallic glass prepared by copper mold casting with reversed pressure. J. Mater. Process. Tech. 2016, 237, 270–276. [Google Scholar] [CrossRef]
- Wang, Y.S.; Tan, M.J.; Chua, B.W.; Bayraktar, E. YSZ-Reinforced Mg-Based Amorphous Composites: Processing, Characterisation & Corrosion. Adv. Mater. Res. 2014, 939, 122–129. [Google Scholar] [CrossRef]
- Wat, A.; Lee, J.I.; Ryu, C.W.; Gludovatz, B.; Kim, J.; Tomsia, A.P.; Ishikawa, T.; Schmitz, J.; Meyer, A.; Alfreider, M.; et al. Bioinspired nacre-like alumina with a bulk-metallic glass-forming alloy as a compliant phase. Nat. Commun. 2019, 10, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Shao, Y.; Gong, P.; Yao, K.F. The effect of simulated thermal cycling on thermal and mechanical stability of a Ti-based bulk metallic glass. J. Alloys Compd. 2013, 575, 449–454. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Gong, P.; Yao, K. Effect of thermal cycling on the mechanical properties of Zr41Ti14Cu12.5Ni10Be22.5 alloy. Sci. China Phys. Mech. 2012, 55, 2357–2361. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Yao, K.-F. Chemical composition dependence of atomic oxygen erosion resistance in Cu-rich bulk metallic glasses. Chin. Sci. Bull. 2012, 57, 4801–4804. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Yang, K.; Bao, X.; Liu, J.; Guo, Q.; Zhang, L.; Wang, Q.; Hua, N. Enhanced wear, corrosion, and corrosive-wear resistance of the biocompatible Ti-based bulk metallic glass by oxidation treatment. J. Non-Cryst. Solids 2022, 576, 121231. [Google Scholar] [CrossRef]
- Jia, Q.; He, W.; Hua, D.; Zhou, Q.; Du, Y.; Ren, Y.; Lu, Z.; Wang, H.; Zhou, F.; Wang, J. Effects of structure relaxation and surface oxidation on nanoscopic wear behaviors of metallic glass. Acta Mater. 2022, 232, 117934. [Google Scholar] [CrossRef]
- Xu, H.; Shao, Y.; Chen, S.; Yao, K. Stress-induced activation of the commercial Fe-based metallic glass ribbons for azo dye degradation. J. Non-Cryst. Solids 2021, 572, 121117. [Google Scholar] [CrossRef]
- Zine, P.U.; Joshi, P.K. Inducing compressive residual stress to minimize effect of crack generation by using controlled shot peening process. Mater. Today Proc. 2023, 72, 870–877. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Shao, Y.; Cheng, M.-T.; Yao, K.-F. Effect of residual stress on azo dye degradation capability of Fe-based metallic glass. J. Non-Cryst. Solids 2017, 473, 74–78. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Zhang, H.; Dan, Z.; Weng, N.; Tang, W.; Qin, F. Superior azo-dye degradation of Fe-Si-B-P amorphous powders with graphene oxide addition. J. Non-Cryst. Solids 2018, 491, 34–42. [Google Scholar] [CrossRef]
- Wu, S.; Pan, Y.; Lu, J.; Wang, N.; Dai, W.; Lu, T. Effect of the addition of Mg, Ti, Ni on the decoloration performance of AlCrFeMn high entropy alloy. J. Mater. Sci. Technol. 2019, 35, 1629–1635. [Google Scholar] [CrossRef]
- Tang, M.; Lai, L.; Ding, D.; Liu, T.; Kang, W.; Guo, N.; Song, B.; Guo, S. Rapid degradation of Direct Blue dye by Co-based amorphous alloy wire. J. Non-Cryst. Solids 2022, 576, 121282. [Google Scholar] [CrossRef]
- Si, J.; Gu, J.; Luan, H.; Yang, X.; Shi, L.; Shao, Y.; Yao, K. Porous composite architecture bestows Fe-based glassy alloy with high and ultra-durable degradation activity in decomposing azo dye. J. Hazard. Mater. 2020, 388, 122043. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Z.; Qin, X.D.; Li, Z.; Zhang, H. Highly efficient and stable CuZr-based metallic glassy catalysts for azo dye degradation. J. Mater. Sci. Technol. 2020, 46, 88–97. [Google Scholar] [CrossRef]
- Peng, X.; Han, J.; Wang, Y.; Bo, Z.; Nie, A.; Li, P.; Li, Y.; Wu, H.; Liu, P.; Lu, Z.; et al. Unexpected enhanced catalytic performance via highly dense interfaces in ultra-fine amorphous-nanocrystalline biphasic structure. Appl. Mater. Today 2022, 29, 101689. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, H.; Yang, X. Efficient degradation of orange II dye using Fe-based metallic glass powders prepared by commercial raw materials. Intermetallics 2021, 129, 107030. [Google Scholar] [CrossRef]
- Miao, F.; Wang, Q.; Di, S.; Yun, L.; Zhou, J.; Shen, B. Enhanced dye degradation capability and reusability of Fe-based amorphous ribbons by surface activation. J. Mater. Sci. Technol. 2020, 53, 163–173. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Lv, M.; Hu, Z. Decolorization of azo dye solution by Fe-Mo-Si-B amorphous alloy. J. Non-Cryst. Solids 2010, 356, 1703–1706. [Google Scholar] [CrossRef]
- Bharti, D.B.; Bharati, A.V. Photocatalytic degradation of Alizarin Red dye under visible light using ZnO & CdO nanomaterial. Optik 2018, 160, 371–379. [Google Scholar] [CrossRef]
- Micheal, K.; Ayeshamariam, A.; Devanesan, S.; Bhuvaneswari, K.; Pazhanivel, T.; AlSalhi, M.S.; Aljaafreh, M.J. Environmental friendly synthesis of carbon nanoplates supported ZnO nanorods for enhanced degradation of dyes and organic pollutants with visible light driven photocatalytic performance. J. King Saud Univ. Sci. 2020, 32, 1081–1087. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Kekedy-Nagy, L.; Teymouri, A.; Herring, A.M.; Greenlee, L.F. Electrochemical removal and recovery of phosphorus as struvite in an acidic environment using pure magnesium vs. the AZ31 magnesium alloy as the anode. Chem. Eng. J. 2020, 380, 122480. [Google Scholar] [CrossRef]
- Yu, B.L.; Uan, J.Y. Sacrificial Mg film anode for cathodic protection of die cast Mg-9 wt.%Al-l wt.%Zn alloy in NaCl aqueous solution. Scr. Mater. 2006, 54, 1253–1257. [Google Scholar] [CrossRef]









| Sample | Nominal Composition (at.%) | Tested Composition (at.%) | ||||
|---|---|---|---|---|---|---|
| Mg | Zn | Ca | Sr | O | ||
| M79 | Mg79Zn17Ca3Sr1 | 78.14 | 16.98 | 2.66 | 0.89 | 1.33 |
| M80 | Mg80Zn16Ca3Sr1 | 79.45 | 15.90 | 2.55 | 0.99 | 1.11 |
| M81 | Mg81Zn15Ca3Sr1 | 80.50 | 15.12 | 2.76 | 0.78 | 0.84 |
| M82 | Mg82Zn14Ca3Sr1 | 81.53 | 13.84 | 2.65 | 0.78 | 1.20 |
| Sample | Tg (K) | Tx (K) | TP1 (K) | Tp2 (K) | Tp3 (K) | Tm (K) | TL (K) | ΔT (K) |
|---|---|---|---|---|---|---|---|---|
| M79 | 346.6 | 382.6 | 394.1 | 507.1 | 539.4 | 632.1 | 679.2 | 36.0 |
| M80 | 347.2 | 380.5 | 394.2 | 504.8 | 538.1 | 628.9 | 682.4 | 33.3 |
| M81 | 349.9 | 379.6 | 389.9 | 503.7 | 543.8 | 629.8 | 686.1 | 29.7 |
| M82 | 351.6 | 379.7 | 391.1 | 503.7 | 550.6 | 629.7 | 687.9 | 28.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Wang, G.; Wang, X.; Yang, W.; Qi, Y. Fast Degradation of Azo Dyes by In Situ Mg-Zn-Ca-Sr Metallic Glass Matrix Composite. Materials 2023, 16, 2201. https://doi.org/10.3390/ma16062201
Jin R, Wang G, Wang X, Yang W, Qi Y. Fast Degradation of Azo Dyes by In Situ Mg-Zn-Ca-Sr Metallic Glass Matrix Composite. Materials. 2023; 16(6):2201. https://doi.org/10.3390/ma16062201
Chicago/Turabian StyleJin, Rui, Gaojiong Wang, Xin Wang, Wei Yang, and Yumin Qi. 2023. "Fast Degradation of Azo Dyes by In Situ Mg-Zn-Ca-Sr Metallic Glass Matrix Composite" Materials 16, no. 6: 2201. https://doi.org/10.3390/ma16062201