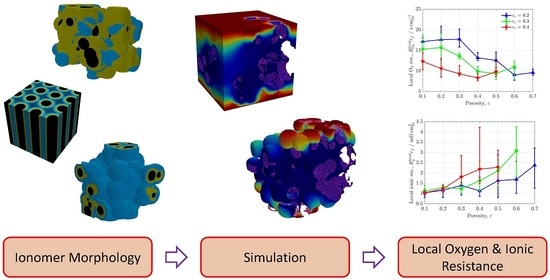
A Numerical Assessment of Mitigation Strategies to Reduce Local Oxygen and Proton Transport Resistances in Polymer Electrolyte Fuel Cells
Abstract
:1. Introduction
2. Methods
2.1. Virtual Reconstruction
- 1.
- Carbon agglomeration. As shown in Figure 1a, spherical carbon particles were randomly located in the domain with a uniform radius of , allowing for overlap between them. Particles were incorporated one by one into the domain until a prescribed carbon volume fraction, , was reached. After each particle addition, only the largest connected component was maintained in the process, while isolated particles not connected to the main carbon structure were removed. At the end, the connectivity of the agglomerated carbon structure to the top and bottom surfaces of the domain was checked, and the generation process was repeated from the beginning if there was not a connected pathway across the domain (six-connected voxels criterion). Usually, no more than five iterations were needed to reach a connected structure at the lowest carbon volume fraction examined, . The generation of the idealized carbon support composed of vertically-aligned cylinders was accomplished using a simplified algorithm. Carbon cylinders were placed with a uniform spacing in the material plane and the radius increased until reaching a prescribed carbon volume fraction.
- 2.
- Ionomer addition. As shown in Figure 1b, a heterogeneous ionomer was created by randomly selecting points from the carbon agglomerate and introducing semi-spherical films around the structure. Ionomer films were incorporated one at a time by identifying the void voxels enclosed in a sphere centered at the selected carbon point with a prescribed ionomer radius, . For each radius, ionomer films were sequentially added until no further variation of the ionomer volume fraction, , was detected (below an established threshold). The whole process was completed when a prescribed porosity, , was reached, gradually increasing by a factor of 1.2 from an exceedingly small value (). For uniform coating, the ionomer phase was simply identified using the Ecludiean distance transform, so that void voxels located at a distance below from the carbon phase were identified as ionomer. As in the heterogeneous case, the ionomer radius was gradually increased by a factor of 1.2 from until reaching the prescribed porosity, . In all cases, connectivity was checked after every ionomer addition to remove isolated components not connected to the main carbon+ionomer structure.
- 3.
- Free water addition. As shown in Figure 1c, free water was added in a similar way to ionomer. However, random points were selected from either carbon, ionomer, or water phases to identify void voxels to be converted into free water. The radius of water spheres, , was sequentially increased by a factor of 1.2 from the last ionomer radius used in Step 2 until reaching a prescribed water saturation, s. In structures with uniform morphology, water was placed uniformly around ionomer by gradually increasing by a factor of 1.2. Isolated water blobs which were not connected to carbon or ionomer were removed.
- (4)
- Additional features. Modifications of the morphology were incorporated to include specific features in the carbon, ionomer, and water distributions. As shown in Figure 1d, meso-porous ionomer was created by introducing water-filled spherical pores in the ionomer phase with a prescribed radius equal to the last ionomer radius used in Step 2. A total of 120 random points were selected from the ionomer phase.
2.2. Numerical Model
3. Discussion of Results
3.1. Calibration
3.2. Volume Composition
3.3. Carbon/Ionomer Interaction
3.4. Ionomer Diffusivity
3.5. Meso-Porous Ionomer
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| A | area/m2 |
| C | species concentration/mol m−3 |
| D | mass diffusivity/m2 s−1 |
| M | molecular mass/kg mol−1 |
| N | flux/mol m−2 s−1 or A m−1 |
| R | universal gas constant/J mol−1 K−1 |
| Ri | mass transport or ionic resistance/s m−1 or Ωm2 |
| r | radius/m |
| rf | roughness factor/− |
| s | water saturation/− |
| T | temperature/K |
| Greek letters | |
| Г | diffusivity or conductivity/IS units |
| δ | thickness/m |
| ε | porosity/− |
| εi | volume fraction of component i/− |
| σp | ionic conductivity/S m−1 |
| ϕp | ionic potential/V |
| φ | transport scalar/IS units |
| Subscripts | |
| c | carbon |
| cl | catalyst layer |
| i | ionomer |
| p | protonic or ionic |
| v | void |
| Superscripts | |
| bulk | bulk property |
| eff | effective |
| kn | Knudsen |
| local | local quantity around active Pt sites |
| w | water |
References
- Fan, L.; Tu, Z.; Chan, S.H. Recent development in design a state-of-art proton exchange membrane fuel cell from stack to system: Theory, integration and prospective. Int. J. Hydrogen Energy 2023, 48, 7828–7865. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Ampah, J.D.; Wilberforce, T.; Afrane, S.; Nutakor, C. Research Progress, Trends, and Current State of Development on PEMFC-New Insights from a Bibliometric Analysis and Characteristics of Two Decades of Research Output. Membranes 2022, 12, 1103. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: An industrial perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Parekh, A. Recent developments of proton exchange membranes for PEMFC: A review. Front. Energy Res. 2022, 10, 956132. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Banham, D.; Zou, J.; Mukerjee, S.; Liu, Z.; Yang, D.; Zhang, Y.; Peng, Y.; Dong, A. Ultralow platinum loading proton exchange membrane fuel cells: Performance losses and solutions. J. Power Sources 2021, 490, 229515. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, S.; Liang, Y.; Li, H.; Xu, Z.; Xu, Q.; Yin, J.; Shen, S.; Yan, X.; Zhang, J. Engineering the catalyst layers towards enhanced local oxygen transport of Low-Pt proton exchange membrane fuel cells: Materials, designs, and methods. Int. J. Hydrogen Energy 2023, 48, 4389–4417. [Google Scholar] [CrossRef]
- Cheng, X.; Shen, S.; Wei, G.; Wang, C.; Luo, L.; Zhang, J. Perspectives on challenges and achievements in local oxygen transport of low Pt proton exchange membrane fuel cells. Adv. Mater. Technol. 2022, 7, 2200228. [Google Scholar] [CrossRef]
- Javed, R.M.N.; Al-Othman, A.; Tawalbeh, M.; Olabi, A.G. Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 2022, 168, 112836. [Google Scholar] [CrossRef]
- Choudhury, F.A.; Norouzi, N.; Amir, K.; Demir, M.; El-Kaderi, H.M. Iron-based sulfur and nitrogen dual doped porous carbon as durable electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 6078–6088. [Google Scholar] [CrossRef]
- García-Salaberri, P.A.; Sánchez-Ramos, A.; Das, P.K. On the optimal cathode catalyst layer for polymer electrolyte fuel cells: Bimodal pore size distributions with functionalized microstructures. Front. Energy Res. 2022, 10, 1058913. [Google Scholar] [CrossRef]
- Weber, A.Z.; Kusoglu, A. Unexplained transport resistances for low-loaded fuel-cell catalyst layers. J. Mater. Chem. A 2014, 2, 17207–17211. [Google Scholar] [CrossRef]
- Sánchez-Ramos, A.; Gostick, J.T.; García-Salaberri, P.A. Modeling the effect of low Pt loading cathode catalyst layer in polymer electrolyte fuel cells: Part I. Model formulation and validation. J. Electrochem. Soc. 2021, 168, 124514. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K.; Schmoeckel, A.K.; Vernstrom, G.D.; Atanasoski, R. High voltage stability of nanostructured thin film catalysts for PEM fuel cells. J. Power Sources 2006, 161, 1002–1011. [Google Scholar] [CrossRef]
- Debe, M.K. Nanostructured thin film electrocatalysts for PEM fuel cells-a tutorial on the fundamental characteristics and practical properties of NSTF catalysts. Ecs Trans. 2012, 45, 47. [Google Scholar] [CrossRef]
- Yu, H.; Roller, J.M.; Mustain, W.E.; Maric, R. Influence of the ionomer/carbon ratio for low-Pt loading catalyst layer prepared by reactive spray deposition technology. J. Power Sources 2015, 283, 84–94. [Google Scholar] [CrossRef]
- Talukdar, K.; Delgado, S.; Lagarteira, T.; Gazdzicki, P.; Friedrich, K.A. Minimizing mass-transport loss in proton exchange membrane fuel cell by freeze-drying of cathode catalyst layers. J. Power Sources 2019, 427, 309–317. [Google Scholar] [CrossRef]
- Conde, J.J.; Folgado, M.A.; Ferreira-Aparicio, P.; Chaparro, A.M.; Chowdhury, A.; Kusoglu, A.; Cullen, D.; Weber, A.Z. Mass-transport properties of electrosprayed Pt/C catalyst layers for polymer-electrolyte fuel cells. J. Power Sources 2019, 427, 250–259. [Google Scholar] [CrossRef]
- Yoshino, S.; Shinohara, A.; Kodama, K.; Morimoto, Y. Fabrication of catalyst layer with ionomer nanofiber scaffolding for polymer electrolyte fuel cells. J. Power Sources 2020, 476, 228584. [Google Scholar] [CrossRef]
- Cheng, X.; You, J.; Shen, S.; Wei, G.; Yan, X.; Wang, C.; Zhang, J. An ingenious design of nanoporous nafion film for enhancing the local oxygen transport in cathode catalyst layers of PEMFCs. Chem. Eng. J. 2022, 439, 135387. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, S.; Shao, P.; Zhu, Y.; Mu, Z.; Sheng, D.; Zhang, T.; Jiang, X.; Shao, R.; Ren, Z.; et al. Covalent organic framework–based porous ionomers for high-performance fuel cells. Science 2022, 378, 181–186. [Google Scholar] [CrossRef]
- Liu, J.; García-Salaberri, P.A.; Zenyuk, I.V. The impact of reaction on the effective properties of multiscale catalytic porous media: A case of polymer electrolyte fuel cells. Transp. Porous Media 2019, 128, 363–384. [Google Scholar] [CrossRef]
- Zenyuk, I.V.; Das, P.K.; Weber, A.Z. Understanding impacts of catalyst-layer thickness on fuel-cell performance via mathematical modeling. J. Electrochem. Soc. 2016, 163, F691. [Google Scholar] [CrossRef]
- Mu, Y.T.; Weber, A.Z.; Gu, Z.L.; Tao, W.Q. Mesoscopic modeling of transport resistances in a polymer-electrolyte fuel-cell catalyst layer: Analysis of hydrogen limiting currents. Appl. Energy 2019, 255, 113895. [Google Scholar] [CrossRef]
- Mu, Y.T.; Yang, S.R.; He, P.; Tao, W.Q. Mesoscopic modeling impacts of liquid water saturation, and platinum distribution on gas transport resistances in a PEMFC catalyst layer. Electrochim. Acta 2021, 388, 138659. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, H.; Yim, S.D.; Sohn, Y.J.; Lee, S.G. Revelation of transport properties of ultra-thin ionomer films in catalyst layer of polymer electrolyte membrane fuel cells using molecular dynamics. Appl. Surf. Sci. 2022, 598, 153815. [Google Scholar] [CrossRef]
- Dou, S.; Hao, L.; Liu, H. Effects of liquid water on the pore structure and transport coefficients in the cathode catalyst layer of PEM fuel cells. Int. J. Hydrogen Energy 2022, 47, 41138–41153. [Google Scholar] [CrossRef]
- Sadeghi, M.A.; Khan, Z.A.; Agnaou, M.; Hu, L.; Litster, S.; Kongkanand, A.; Padgett, E.; Muller, D.A.; Friscic, T.; Gostick, J. Predicting pemfc performance from a volumetric image of catalyst layer structure using pore network modeling. Appl. Energy 2024, 353, 122004. [Google Scholar] [CrossRef]
- Mu, Y.T.; He, P.; Gu, Z.L.; Qu, Z.G.; Tao, W.Q. Modelling the reactive transport processes in different reconstructed agglomerates of a PEFC catalyst layer. Electrochim. Acta 2022, 404, 139721. [Google Scholar] [CrossRef]
- Dou, S.; Hao, L.; Liu, H. Effects of carbon aggregates and ionomer distribution on the performance of PEM fuel cell catalyst layer: A pore-scale study. Renew. Energy 2023, 217, 119254. [Google Scholar] [CrossRef]
- García-Salaberri, P.A.; Hwang, G.; Vera, M.; Weber, A.Z.; Gostick, J.T. Effective diffusivity in partially-saturated carbon-fiber gas diffusion layers: Effect of through-plane saturation distribution. Int. J. Heat Mass Transf. 2015, 86, 319–333. [Google Scholar] [CrossRef]
- Hack, J.; García-Salaberri, P.A.; Kok, M.D.; Jervis, R.; Shearing, P.R.; Brandon, N.; Brett, D.J. X-ray micro-computed tomography of polymer electrolyte fuel cells: What is the representative elementary area? J. Electrochem. Soc. 2020, 167, 013545. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, G.; Wang, C.; Shen, S.; Zhang, J. Experimental probing of effects of carbon support on bulk and local oxygen transport resistance in ultra-low Pt PEMFCs. Int. J. Heat Mass Transf. 2021, 164, 120549. [Google Scholar] [CrossRef]
- Kulikovsky, A. The effect of Nafion film on the cathode catalyst layer performance in a low—Pt PEM fuel cell. Electrochem. Commun. 2019, 103, 61–65. [Google Scholar] [CrossRef]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. Oxygen solubility, diffusion coefficient, and solution viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Gostick, J.T.; Weber, A.Z. Resistor-network modeling of ionic conduction in polymer electrolytes. Electrochim. Acta 2015, 179, 137–145. [Google Scholar] [CrossRef]
- Ohira, A.; Kuroda, S.; Mohamed, H.F.; Tavernier, B. Effect of interface on surface morphology and proton conduction of polymer electrolyte thin films. Phys. Chem. Chem. Phys. 2013, 15, 11494–11500. [Google Scholar] [CrossRef]
- Chen, D.; Kongkanand, A.; Jorne, J. Proton conduction and oxygen diffusion in ultra-thin nafion films in PEM fuel cell: How thin? J. Electrochem. Soc. 2019, 166, F24. [Google Scholar] [CrossRef]
- Paul, D.K.; McCreery, R.; Karan, K. Proton transport property in supported Nafion nanothin films by electrochemical impedance spectroscopy. J. Electrochem. Soc. 2014, 161, F1395. [Google Scholar] [CrossRef]
- Zenyuk, I.V.; Litster, S. Modeling ion conduction and electrochemical reactions in water films on thin-film metal electrodes with application to low temperature fuel cells. Electrochim. Acta 2014, 146, 194–206. [Google Scholar] [CrossRef]
- Liu, J.; Zenyuk, I.V. Proton transport in ionomer-free regions of polymer electrolyte fuel cells and implications for oxygen reduction reaction. Curr. Opin. Electrochem. 2018, 12, 202–208. [Google Scholar] [CrossRef]
- Sánchez-Ramos, A.; Gostick, J.T.; García-Salaberri, P.A. Modeling the effect of low pt loading cathode catalyst layer in polymer electrolyte fuel cells. part ii: Parametric analysis. J. Electrochem. Soc. 2022, 169, 074503. [Google Scholar] [CrossRef]
- Yoon, W.; Weber, A.Z. Modeling low-platinum-loading effects in fuel-cell catalyst layers. J. Electrochem. Soc. 2011, 158, B1007. [Google Scholar] [CrossRef]
- Petrovick, J.G.; Radke, C.J.; Weber, A.Z. Gas Mass-Transport Coefficients in Ionomer Membranes Using a Microelectrode. ACS Meas. Sci. 2022, 2, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- García-Salaberri, P.A. Proton exchange membranes for polymer electrolyte fuel cells: An analysis of perfluorosulfonic acid and aromatic hydrocarbon ionomers. Sustain. Mater. Technol. 2023, 38, e00727. [Google Scholar] [CrossRef]
- Greszler, T.A.; Caulk, D.; Sinha, P. The impact of platinum loading on oxygen transport resistance. J. Electrochem. Soc. 2012, 159, F831. [Google Scholar] [CrossRef]
- Schuler, T.; Chowdhury, A.; Freiberg, A.T.; Sneed, B.; Spingler, F.B.; Tucker, M.C.; More, K.L.; Radke, C.J.; Weber, A.Z. Fuel-cell catalyst-layer resistance via hydrogen limiting-current measurements. J. Electrochem. Soc. 2019, 166, F3020–F3031. [Google Scholar] [CrossRef]
- Owejan, J.P.; Owejan, J.E.; Gu, W. Impact of platinum loading and catalyst layer structure on PEMFC performance. J. Electrochem. Soc. 2013, 160, F824. [Google Scholar] [CrossRef]
- Sun, X.; Yu, H.; Zhou, L.; Gao, X.; Zeng, Y.; Yao, D.; He, L.; Shao, Z. Influence of platinum dispersity on oxygen transport resistance and performance in PEMFC. Electrochim. Acta 2020, 332, 135474. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, X.; Yan, X.; Shen, S.; Ke, C.; Wei, G.; Zhang, J. Respective influence of ionomer content on local and bulk oxygen transport resistance in the catalyst layer of PEMFCs with low Pt loading. J. Electrochem. Soc. 2019, 166, F239. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Kumaraguru, S.; Koestner, R.; Fuller, T.; Gu, W.; Kariuki, N.; Myers, D.; Dudenas, P.J.; Kusoglu, A. Editors’ choice—Ionomer side chain length and equivalent weight impact on high current density transport resistances in PEMFC cathodes. J. Electrochem. Soc. 2021, 168, 024518. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Gu, W.; Ziegelbauer, J.M.; Kumaraguru, S. Carbon support microstructure impact on high current density transport resistances in PEMFC cathode. J. Electrochem. Soc. 2020, 167, 064515. [Google Scholar] [CrossRef]
- Orfanidi, A.; Madkikar, P.; El-Sayed, H.A.; Harzer, G.S.; Kratky, T.; Gasteiger, H. The key to high performance low Pt loaded electrodes. J. Electrochem. Soc. 2017, 164, F418. [Google Scholar] [CrossRef]
- Ünsal, S.; Bozzetti, M.; Chen, Y.C.; Girod, R.; Berger, A.; Diercks, J.S.; Gialamoidou, S.; Lyu, J.; Medarde, M.; Gasteiger, H.A.; et al. Catalyst Aggregate Size Effect on the Mass Transport Properties of Non-Noble Metal Catalyst Layers for PEMFC Cathodes. J. Electrochem. Soc. 2023, 170, 074502. [Google Scholar] [CrossRef]
- Liu, Y.; Murphy, M.W.; Baker, D.R.; Gu, W.; Ji, C.; Jorne, J.; Gasteiger, H.A. Proton conduction and oxygen reduction kinetics in PEM fuel cell cathodes: Effects of ionomer-to-carbon ratio and relative humidity. J. Electrochem. Soc. 2009, 156, B970. [Google Scholar] [CrossRef]
- Morawietz, T.; Handl, M.; Oldani, C.; Friedrich, K.A.; Hiesgen, R. Quantitative in situ analysis of ionomer structure in fuel cell catalytic layers. ACS Appl. Mater. Interfaces 2016, 8, 27044–27054. [Google Scholar] [CrossRef]
- Suzuki, T.; Okada, S.; Tsushima, S. Analysis of ionomer distribution and Pt/C agglomerate size in catalyst layers by two-stage ion-beam processing. J. Electrochem. Soc. 2020, 167, 124513. [Google Scholar] [CrossRef]
- Normile, S.J.; Zenyuk, I.V. Imaging ionomer in fuel cell catalyst layers with synchrotron nano transmission x-ray microscopy. Solid State Ionics 2019, 335, 38–46. [Google Scholar] [CrossRef]
- Sabarirajan, D.C.; Liu, J.; Qi, Y.; Perego, A.; Haug, A.T.; Zenyuk, I.V. Determining proton transport in pseudo catalyst layers using hydrogen pump DC and AC techniques. J. Electrochem. Soc. 2020, 167, 084521. [Google Scholar] [CrossRef]
- Harzer, G.S.; Orfanidi, A.; El-Sayed, H.; Madkikar, P.; Gasteiger, H.A. Tailoring catalyst morphology towards high performance for low Pt loaded PEMFC cathodes. J. Electrochem. Soc. 2018, 165, F770. [Google Scholar] [CrossRef]
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hübner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Imanishi, M.; Hasegawa, S.; Namba, R. Vertically aligned carbon nanotube electrodes for high current density operating proton exchange membrane fuel cells. J. Power Sources 2014, 253, 104–113. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, S.; Jiang, L.; Sun, H.; Liu, S.; Fu, X.; Zhang, B.; Sheng Su, D.; Wang, J.; Sun, G. Bio-inspired construction of advanced fuel cell cathode with Pt anchored in ordered hybrid polymer matrix. Sci. Rep. 2015, 5, 16100. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.H.; Hao, C.; Yan, B.; Yang, B.; Liu, J.; Shen, P.K.; Tian, Z.Q. High-performance proton exchange membrane fuel cell with ultra-low loading Pt on vertically aligned carbon nanotubes as integrated catalyst layer. J. Energy Chem. 2022, 71, 497–506. [Google Scholar] [CrossRef]
- Yoshimune, W. Dependence of oxygen transport properties of catalyst layers for polymer electrolyte fuel cells on the fabrication process. Results Chem. 2023, 5, 100738. [Google Scholar] [CrossRef]
- Colombo, E.; Baricci, A.; Bisello, A.; Guetaz, L.; Casalegno, A. PEMFC performance decay during real-world automotive operation: Evincing degradation mechanisms and heterogeneity of ageing. J. Power Sources 2023, 553, 232246. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J.H.; Park, J.S. Effect of Blended Perfluorinated Sulfonic Acid Ionomer Binder on the Performance of Catalyst Layers in Polymer Electrolyte Membrane Fuel Cells. Membranes 2023, 13, 794. [Google Scholar] [CrossRef]
- Hutapea, Y.A.; Nishihara, M.; Gautama, Z.A.R.; Mufundirwa, A.; Lyth, S.M.; Sugiyama, T.; Nagayama, M.; Sasaki, K.; Hayashi, A. Reduction of oxygen transport resistance in PEFC cathode through blending a high oxygen permeable polymer. J. Power Sources 2023, 556, 232500. [Google Scholar] [CrossRef]
- Fang, S.; Liu, G.; Li, M.; Zhang, H.; Yu, J.; Zhang, F.; Pan, M.; Tang, H. Tailoring Ionomer Chemistry for Improved Oxygen Transport in the Cathode Catalyst Layer of Proton Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2023, 6, 3590–3598. [Google Scholar] [CrossRef]
- Braaten, J.P.; Kariuki, N.N.; Myers, D.J.; Blackburn, S.; Brown, G.; Park, A.; Litster, S. Integration of a high oxygen permeability ionomer into polymer electrolyte membrane fuel cell cathodes for high efficiency and power density. J. Power Sources 2022, 522, 230821. [Google Scholar] [CrossRef]
- Doo, G.; Yuk, S.; Lee, J.H.; Choi, S.; Lee, D.H.; Lee, D.W.; Hyun, J.; Kwon, S.H.; Lee, S.G.; Kim, H.T. Nano-scale control of the ionomer distribution by molecular masking of the Pt surface in PEMFCs. J. Mater. Chem. A 2020, 8, 13004–13013. [Google Scholar] [CrossRef]
- Lee, J.H.; Doo, G.; Kwon, S.H.; Kang, H.; Choi, S.; Yim, S.D.; Kim, H.T.; Lee, S.G. Controlling ionomer film morphology through altering Pt catalyst surface properties for polymer electrolyte membrane fuel cells. ACS Appl. Polym. Mater. 2020, 2, 1807–1818. [Google Scholar] [CrossRef]








| Ionomer Morphology | Local O2 Resistance | Local Ionic Resistance |
|---|---|---|
| Heterogeneous ( 0.1–0.3) | 14.88 (–) | 1.21 (–) |
| Heterogeneous ( 0.4–0.6) | 10.09 (−32.19%) | 2.27 (+87.6%) |
| Uniform ( 0.1–0.4) | 14.03 (−5.71%) | 1.23 (+1.65%) |
| Uniform ( 0.5–0.6) | 4.57 (−69.29%) | 1.83 (+51.24%) |
| Ideal ( 0.1–0.6) | 3.42 (−77.02%) | 0.82 (−31.92%) |
| Diffusivity increase ( 0.1–0.6) | 5.93 (−60.15%) | 1.64 (+35.9%) |
| Meso-porous ( 0.1–0.6) | 0.43 (−97.08%) | 2.58 (+113.22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Salaberri, P.A. A Numerical Assessment of Mitigation Strategies to Reduce Local Oxygen and Proton Transport Resistances in Polymer Electrolyte Fuel Cells. Materials 2023, 16, 6935. https://doi.org/10.3390/ma16216935
García-Salaberri PA. A Numerical Assessment of Mitigation Strategies to Reduce Local Oxygen and Proton Transport Resistances in Polymer Electrolyte Fuel Cells. Materials. 2023; 16(21):6935. https://doi.org/10.3390/ma16216935
Chicago/Turabian StyleGarcía-Salaberri, Pablo A. 2023. "A Numerical Assessment of Mitigation Strategies to Reduce Local Oxygen and Proton Transport Resistances in Polymer Electrolyte Fuel Cells" Materials 16, no. 21: 6935. https://doi.org/10.3390/ma16216935