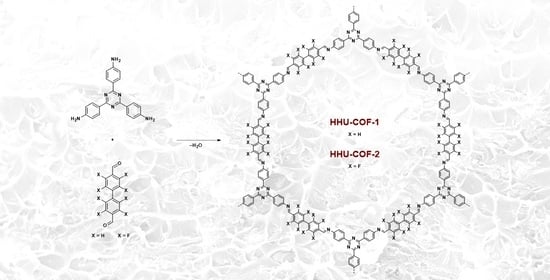
Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HHU-COF-1
2.3. Synthesis of HHU-COF-1 (Larger Scale)
2.4. Synthesis of HHU-COF-2
2.5. Synthesis of HHU-COF-2 (Larger Scale)
2.6. Preparation of HHU-COF-1/Matrimid and HHU-COF-2/Matrimid MMMs
2.7. Instrumentation and Characterization Methods
3. Results and Discussion
3.1. Characterization of HHU-COF-1 and HHU-COF-2
3.2. Characterization of MMMs
3.3. Gas Permeability and Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Geng, K.; Liu, R.; Tan, K.T.; Gong, Y.; Li, Z.; Tao, S.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Chemical Approaches to Designer Structures and Built-In Functions. Angew. Chem. Int. Ed. 2020, 59, 5050–5091. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, H.; Ma, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 2019, 11, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Wheatley, P.S. Gas Storage in Nanoporous Materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M.; et al. Recent advances in covalent organic frameworks (COFs) as a smart sensing material. Chem. Soc. Rev. 2019, 48, 5266–5302. [Google Scholar] [CrossRef]
- Hu, Y.; Dunlap, N.; Wan, S.; Lu, S.; Huang, S.; Sellinger, I.; Ortiz, M.; Jin, Y.; Lee, S.-H.; Zhang, W. Crystalline Lithium Imidazolate Covalent Organic Frameworks with High Li-Ion Conductivity. J. Am. Chem. Soc. 2019, 141, 7518–7525. [Google Scholar] [CrossRef]
- Keller, N.; Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 2021, 50, 1813–1845. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klöck, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki–Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Gomes, R.; Bhanja, P.; Bhaumik, A. A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem. Commun. 2015, 51, 10050–10053. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Ke, C.; Huang, X.; Zhang, G.; Zhang, Q.; Zhang, Z.; Zhang, Y.; Liu, Y.; Ning, F.; Xi, K. Catalyst-free and efficient fabrication of highly crystalline fluorinated covalent organic frameworks for selective guest adsorption. J. Mater. Chem. A 2019, 7, 18959–18970. [Google Scholar] [CrossRef]
- Wessely, I.D.; Schade, A.M.; Dey, S.; Bhunia, A.; Nuhnen, A.; Janiak, C.; Bräse, S. Covalent Triazine Frameworks Based on the First Pseudo-Octahedral Hexanitrile Monomer via Nitrile Trimerization: Synthesis, Porosity, and CO2 Gas Sorption Properties. Materials 2021, 14, 3214. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Zhao, S.; Van Der Voort, P. Newly Designed Covalent Triazine Framework Based on Novel N-Heteroaromatic Building Blocks for Efficient CO2 and H2 Capture and Storage. ACS Appl. Mater. Interfaces 2018, 10, 1244–1249. [Google Scholar] [CrossRef]
- Bhunia, A.; Boldog, I.; Möller, A.; Janiak, C. Highly stable nanoporous covalent triazine-based frameworks with an adamantane core for carbon dioxide sorption and separation. J. Mater. Chem. A 2013, 1, 14990–14999. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, D.; Huang, D.; Zeng, G.; Xu, P.; Lai, C.; Chen, M.; Cheng, M.; Zhang, C.; Wang, Z. Covalent triazine frameworks for carbon dioxide capture. J. Mater. Chem. A 2019, 7, 22848–22870. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Belmabkhout, Y.; Cadiau, A.; Adil, K.; Shekhah, O.; Shkurenko, A.; Barbour, L.J.; Eddaoudi, M. A Fine-Tuned Fluorinated MOF Addresses the Needs for Trace CO2 Removal and Air Capture Using Physisorption. J. Am. Chem. Soc. 2016, 138, 9301–9307. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-S.; Chang, Z.; Li, Y.-F.; Jiang, Z.-Y.; Xuan, Z.-H.; Zhang, Y.-H.; Li, J.-R.; Chen, Q.; Hu, T.-L.; Bu, X.-H. Fluorous Met-al-Organic Frameworks with Enhanced Stability and High H2/CO2 Storage Capacities. Sci. Rep. 2013, 3, 3312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Yao, K.X.; Teng, B.Y.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water–tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Jena, H.S.; Krishnaraj, C.; Zhao, S.; Depauw, H.; Tahir, N.; Liu, Y.-Y.; Van Der Voort, P. A fluorine-containing hydrophobic covalent triazine framework with excellent selective CO2 capture performance. J. Mater. Chem. A 2018, 6, 6370–6375. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Breitzke, H.; Groszewicz, P.B.; Buntkowsky, G.; Janiak, C. Two linkers are better than one: Enhancing CO2 capture and separation with porous covalent triazine-based frameworks from mixed nitrile linkers. J. Mater. Chem. A 2017, 5, 3609–3620. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Dechnik, J.; Sumby, C.J.; Janiak, C. Enhancing Mixed-Matrix Membrane Performance with Metal-Organic Framework Additives. Cryst. Growth Des. 2017, 17, 4467–4488. [Google Scholar] [CrossRef] [Green Version]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wang, J.; Li, X. Two-Dimensional Covalent Organic Frameworks (COFs) for Membrane Separation: A Mini Review. Ind. Eng. Chem. Res. 2019, 58, 15394–15406. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. [Google Scholar] [CrossRef]
- Dey, S.; Bügel, S.; Sorribas, S.; Nuhnen, A.; Bhunia, A.; Coronas, J.; Janiak, C. Synthesis and Characterization of Covalent Triazine Framework CTF-1@Polysulfone Mixed Matrix Membranes and Their Gas Separation Studies. Front. Chem. 2019, 7, 693. [Google Scholar] [CrossRef]
- Shan, M.; Seoane, B.; Rozhko, E.; Dikhtiarenko, A.; Clet, G.; Kapteijn, F.; Gascon, J. Azine-Linked Covalent Organic Framework (COF)-Based Mixed-Matrix Membranes for CO2/CH4 Separation. Chem. Eur. J. 2016, 22, 14467–14470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, J.; Huang, T.; Xue, J.; Ren, Y.; Guo, Z.; Wang, H.; Yang, L.; Yin, Y.; Jiang, Z.; et al. Mixed-Matrix Membranes with Covalent Triazine Framework Fillers in Polymers of Intrinsic Microporosity for CO2 Separations. Ind. Eng. Chem. Res. 2019, 59, 5296–5306. [Google Scholar] [CrossRef]
- Kunde, T.; Pausch, T.; Reiss, G.J.; Schmidt, B.M. Highly Fluorinated Trianglimine Macrocycles: A Supramolecular Organic Framework. Synlett 2022, 33, 161–165. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Zou, Y.; Zhang, Y.; Xia, H.; Liu, X.; Mu, Y. A 2D azine-linked covalent organic framework for gas storage ap-plications. Chem. Commun. 2014, 50, 13825–13828. [Google Scholar] [CrossRef] [PubMed]
- Loloei, M.; Moghadassi, A.; Omidkhah, M.; Amooghin, A.E. Improved CO2 separation performance of Matrimid®5218 membrane by addition of low molecular weight polyethylene glycol. Greenh. Gas. Sci. Technol. 2015, 5, 530–544. [Google Scholar] [CrossRef]
- Bügel, S.; Hoang, Q.-D.; Spieß, A.; Sun, Y.; Xing, S.; Janiak, C. Biphenyl-Based Covalent Triazine Framework/Matrimid® Mixed-Matrix Membranes for CO2/CH4 Separation. Membranes 2021, 11, 795. [Google Scholar] [CrossRef]
- Jaeger, C.; Hemmann, F. EASY: A simple tool for simultaneously removing background, deadtime and acoustic ringing in quantitative NMR spectroscopy—Part I: Basic principle and applications. Solid State Nucl. Magn. Reson. 2014, 57–58, 22–28. [Google Scholar] [CrossRef]
- Mullangi, D.; Shalini, S.; Nandi, S.; Choksi, B.; Vaidhyanathan, R. Super-hydrophobic covalent organic frameworks for chemical resistant coatings and hydrophobic paper and textile composites. J. Mater. Chem. A 2017, 5, 8376–8384. [Google Scholar] [CrossRef]
- Chen, T.; Li, W.-Q.; Hu, W.-B.; Hu, W.-J.; Liu, Y.A.; Yang, H.; Wen, K. Direct synthesis of covalent triazine-based frameworks (CTFs) through aromatic nucleophilic substitution reactions. RSC Adv. 2019, 9, 18008–18012. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Goodeal, N.; Chen, Y.; Ganose, A.M.; Palgrave, R.G.; Bronstein, H.; Blunt, M.O. Probing the chemical structure of monolayer covalent-organic frameworks grown via Schiff-base condensation reactions. Chem. Commun. 2016, 52, 9941–9944. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, Z.; Do-Thanh, C.-L.; Dai, S. What Fluorine Can Do in CO2 Chemistry: Applications from Homogeneous to Heterogeneous Systems. ChemSusChem 2020, 13, 6182–6200. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Paul, D.R. Physical aging of thin glassy polymer films: Free volume interpretation. J. Membr. Sci. 2006, 277, 219–229. [Google Scholar] [CrossRef]
- Kanehashi, S.; Chen, G.Q.; Scholes, C.A.; Ozcelik, B.; Hua, C.; Ciddor, L.; Southon, P.D.; D’Alessandro, D.M.; Kentish, S.E. Enhancing gas permeability in mixed matrix membranes through tuning the nanoparticle properties. J. Membr. Sci. 2015, 482, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Bouma, R.H.B.; Checchetti, A.; Chidichimo, G.; Drioli, E. Permeation through a heterogeneous membrane: The effect of the dispersed phase. J. Membr. Sci. 1997, 128, 141–149. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Hashin, Z.; Shtrikman, S.A. Variational Approach to the Theory of the Effective Magnetic Permeability of Multiphase Materials. J. Appl. Phys. 1962, 33, 3125–3131. [Google Scholar] [CrossRef]
- Bügel, S.; Spieß, A.; Janiak, C. Covalent triazine framework CTF-fluorene as porous filler material in mixed matrix membranes for CO2/CH4 separation. Micropor. Mesopor. Mater. 2021, 316, 110941. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, L.; Ying, Y.; Wang, Y.; Liu, G.; Dong, J.; Ng, D.Z.L.; Khan, S.A.; Zhao, D. Highly efficient CO2 capture by mixed matrix membranes containing three-dimensional covalent organic framework fillers. J. Mater. Chem. A 2019, 7, 4549–4560. [Google Scholar] [CrossRef]
- Thankamony, R.L.; Li, X.; Das, S.K.; Ostwal, M.M.; Lai, Z. Porous covalent triazine piperazine polymer (CTPP)/PEBAX mixed matrix membranes for CO2/N2 and CO2/CH4 separations. J. Membr. Sci. 2019, 591, 117348. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Wang, S.; Peng, D.; Yang, L.; Wu, Y.; Xin, Q.; Wu, H.; Jiang, Z. Mixed matrix membranes comprising polymers of intrinsic microporosity and covalent organic framework for gas separation. J. Membr. Sci. 2017, 528, 273–283. [Google Scholar] [CrossRef]
- Chen, T.-H.; Popov, I.; Zenasni, O.; Daugulis, O.; Miljanić, O.Š. Superhydrophobic perfluorinated metal–organic frameworks. Chem. Commun. 2013, 49, 6846–6848. [Google Scholar] [CrossRef] [Green Version]
- de la Peña Ruigómez, A.; Rodríguez-San-Miguel, D.; Stylianou, K.C.; Cavallini, M.; Gentili, D.; Liscio, F.; Milita, S.; Roscioni, O.M.; Ruiz-González, M.L.; Carbonell, C.; et al. Direct On-Surface Patterning of a Crystalline Laminar Covalent Organic Framework Synthesized at Room Temperature. Chem. Eur. J. 2015, 21, 10666–10670. [Google Scholar] [CrossRef]
- Huang, N.; Chen, X.; Krishna, R.; Jiang, D. Two-Dimensional Covalent Organic Frameworks for Carbon Dioxide Capture through Channel-Wall Functionalization. Angew. Chem. Int. Ed. 2015, 54, 2986–2990. [Google Scholar] [CrossRef] [Green Version]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of Chemical Stability and Crystallinity in Porphyrin-Containing Covalent Organic Frameworks by Intramolecular Hydrogen Bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056. [Google Scholar] [CrossRef]
- Kaleeswaran, D.; Vishnoi, P.; Murugavel, R. [3+3] Imine and β-ketoenamine tethered fluorescent covalent-organic frameworks for CO2 uptake and nitroaromatic sensing. J. Mater. Chem. C 2015, 3, 7159–7171. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]











| COF | C (wt%) | H (wt%) | N (wt%) |
|---|---|---|---|
| HHU-COF-1 Calculated | 81.93 | 4.42 | 13.65 |
| HHU-COF-1 | 81.09 | 4.27 | 13.17 |
| HHU-COF-2 Calculated a | 60.66 | 1.82 | 10.11 |
| HHU-COF-2 b | 60.94 | 1.93 | 10.35 |
| Filler Material | Filler Content (wt%) | P CO2 (Barrer) | P CH4 (Barrer) | α CO2/CH4 |
|---|---|---|---|---|
| - | 0 | 6.8 ± 0.3 | 0.16 ± 0.01 | 42 ± 1 |
| HHU-COF-1 | 8 | 9.1 ± 0.2 | 0.19 ± 0.01 | 46 ± 2 |
| 16 | 9.1 ± 1.0 | 0.20 ± 0.02 | 46 ± 1 | |
| 24 | 5.8 ± 0.7 | 0.14 ± 0.02 | 41 ± 1 | |
| HHU-COF-2 | 8 | 7.1 ± 0.3 | 0.14 ± 0.01 | 51 ± 1 |
| 16 | 10.2 ± 0.3 | 0.23 ± 0.02 | 44 ± 2 | |
| 24 | 13.0 ± 1.0 | 0.32 ± 0.02 | 40 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bügel, S.; Hähnel, M.; Kunde, T.; de Sousa Amadeu, N.; Sun, Y.; Spieß, A.; Beglau, T.H.Y.; Schmidt, B.M.; Janiak, C. Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation. Materials 2022, 15, 2807. https://doi.org/10.3390/ma15082807
Bügel S, Hähnel M, Kunde T, de Sousa Amadeu N, Sun Y, Spieß A, Beglau THY, Schmidt BM, Janiak C. Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation. Materials. 2022; 15(8):2807. https://doi.org/10.3390/ma15082807
Chicago/Turabian StyleBügel, Stefanie, Malte Hähnel, Tom Kunde, Nader de Sousa Amadeu, Yangyang Sun, Alex Spieß, Thi Hai Yen Beglau, Bernd M. Schmidt, and Christoph Janiak. 2022. "Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation" Materials 15, no. 8: 2807. https://doi.org/10.3390/ma15082807