Investigation on the Adsorption-Interaction Mechanism of Pb(II) at Surface of Silk Fibroin Protein-Derived Hybrid Nanoflower Adsorbent
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Synthesis of SF@Cu-HNFs via Self-Assembly
2.3. Characterization of SF@Cu-HNFs
2.4. Adsorption Performances of SF@Cu-NFs
2.4.1. Adsorption Kinetics Experiment
2.4.2. Adsorption Isotherm Experiment and Adsorption Thermodynamics
3. Results and Discussion
3.1. Condition Optimization for SF@Cu-NFs Preparation
3.1.1. Effect of SF Protein on SF@Cu-NFs Formation
3.1.2. Effect of SF Concentration on SF@Cu-NFs Formation
3.1.3. Effect of Reaction Time on SF@Cu-NFs Formation
3.2. Characterization of SF@Cu-NFs Product
3.2.1. Surface Morphology Measurement by LSCM and SEM Image
3.2.2. Chemical Structure Investigation by FTIR and XRD
3.2.3. Component Analysis by TGA
3.3. SF@Cu-NFs Adsorption Performance for Pb(II)
3.3.1. Investigation of pH Influence on Pb(II) Adsorption
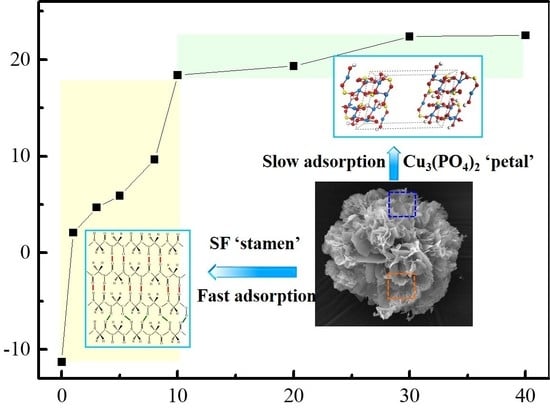
3.3.2. Evaluation of Adsorption Kinetics
3.3.3. Adsorption Isotherm Experiment and Adsorption Thermodynamics
3.3.4. Investigation of Adsorption Selectivity for Pb(II)
3.4. SF@Cu-NFs Adsorption Mechanism for Pb(II)
3.4.1. Verification of Pb(II) Adsorption by SF@Cu-NFs
3.4.2. Mechanism Analysis of Pb(II) Adsorption by SF@Cu-NFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water-An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Needleman, H. Lead poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Ilbeigi, V.; Valadbeigi, Y.; Tabrizchi, M. Ion Mobility Spectrometry of Heavy Metals. Anal. Chem. 2016, 88, 7324–7328. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Moyo, M.; Guyo, U.; Mawenyiyo, G.; Zinyama, N.P.; Nyamunda, B.C. Marula seed husk (Sclerocarya birrea) biomass as a low cost biosorbent for removal of Pb(II) and Cu(II) from aqueous solution. J. Ind. Eng. Chem. 2015, 27, 126–132. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Xia, L.; Yin, H.; García Meza, J.V.; Ju, W. Enhanced Pb(II) removal by algal-based biosorbent cultivated in high-phosphorus cultures. Chem. Eng. J. 2019, 361, 167–179. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling-an overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Hao, T.-w.; Xiang, P.-y.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.-K.; van Loosdrecht, M.C.M.; Chen, G.-H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Bolisetty, S.; Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, J.; Wang, L.; Liu, X.; Meng, J.; Tang, X.; Tang, C.; Xu, J. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem. Eng. J. 2019, 358, 1399–1409. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, P.; Liu, J.; Lin, Y.; Cai, Y.; Ye, Y.; Liang, C. Facet-Dependent Selective Adsorption of Mn-Doped α-Fe2O3 Nanocrystals toward Heavy-Metal Ions. Chem. Mater. 2017, 29, 10198–10205. [Google Scholar] [CrossRef]
- Lagadic, I.L.; Mitchell, M.K.; Payne, B.D. Highly Effective Adsorption of Heavy Metal Ions by a Thiol-Functionalized Magnesium Phyllosilicate Clay. Environ. Sci. Technol. 2001, 35, 984–990. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Qu, J.; Yan, W.-S.; Zhu, J.-F.; Wu, Z.-Y.; Song, W.-G. Low-Cost Synthesis of Flowerlike α-Fe2O3 Nanostructures for Heavy Metal Ion Removal: Adsorption Property and Mechanism. Langmuir 2012, 28, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Ide, Y.; Ochi, N.; Ogawa, M. Effective and Selective Adsorption of Zn2+ from Seawater on a Layered Silicate. Angew. Chem. Int. Ed. 2011, 50, 654–656. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Zheng, L.; Dang, Z.; Zhang, L. Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem. Eng. J. 2018, 350, 1000–1009. [Google Scholar] [CrossRef]
- Sellaoui, L.; Soetaredjo, F.E.; Ismadji, S.; Bonilla-Petriciolet, A.; Belver, C.; Bedia, J.; Ben Lamine, A.; Erto, A. Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems. Chem. Eng. J. 2018, 354, 569–576. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Ali, O.; Wei, Y.; Zhang, S.; Zhang, Y.; Cai, T.; Liu, C.; Luo, S. Fast adsorption of heavy metal ions by waste cotton fabrics based double network hydrogel and influencing factors insight. J. Hazard. Mater. 2018, 344, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Di Toro, D.M.; Allen, H.E.; Sparks, D.L. A general model for kinetics of heavy metal adsorption and desorption on soils. Environ. Sci. Technol. 2013, 47, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight Studies on Metal-Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Z.; Yuan, Y.; Guo, Z. Experimental study on functional graphene oxide containing many primary amino groups fast-adsorbing heavy metal ions and adsorption mechanism. Sep. Sci. Technol. 2018, 53, 1666–1677. [Google Scholar] [CrossRef]
- Kampalanonwat, P.; Supaphol, P. Preparation and Adsorption Behavior of Aminated Electrospun Polyacrylonitrile Nanofiber Mats for Heavy Metal Ion Removal. ACS Appl. Mater. Interfaces 2010, 2, 3619–3627. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvasa, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Wei, F.; Qu, J.; Song, W.-G. Programmed Synthesis of Magnetic Magnesium Silicate Nanotubes with High Adsorption Capacities for Lead and Cadmium Ions. Chem. A Eur. J. 2013, 19, 1558–1562. [Google Scholar] [CrossRef]
- Negron, L.M.; Diaz, T.L.; Ortiz-Quiles, E.O.; Dieppa-Matos, D.; Madera-Soto, B.; Rivera, J.M. Organic Nanoflowers from a Wide Variety of Molecules Templated by a Hierarchical Supramolecular Scaffold. Langmuir 2016, 32, 2283–2290. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Yang, Y.; Huang, C.; Huang, M.; Chen, J.; Rao, T.; Ran, X. Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide. J. Hazard. Mater. 2019, 373, 131–140. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, D.-P.; Ye, D.; Zhang, X.; Fang, X.; Zhang, S.; Liu, B.; Kong, J. Protein-inorganic hybrid nanoflowers as ultrasensitive electrochemical cytosensing Interfaces for evaluation of cell surface sialic acid. Biosens. Bioelectron. 2015, 68, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, J.; Liu, J.; Zhang, H.; Jiang, J.; Yu, R. A dual enzyme-inorganic hybrid nanoflower incorporated microfluidic paper-based analytic device (mu PAD) biosensor for sensitive visualized detection of glucose. Nanoscale 2017, 9, 5658–5663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wen, M.; Wen, W.; Du, D.; Zhang, X.; Wang, S.; Lin, Y. Recent progress in biosensors based on organic–inorganic hybrid nanoflowers. Biosens. Bioelectron. 2018, 120, 175–187. [Google Scholar] [CrossRef]
- Thawari, A.G.; Rao, C. Peroxidase-like Catalytic Activity of Copper-Mediated Protein-Inorganic Hybrid Nanoflowers and Nanofibers of beta-Lactoglobulin and alpha-Lactalbumin: Synthesis, Spectral Characterization, Microscopic Features, and Catalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 10392–10402. [Google Scholar] [CrossRef] [Green Version]
- Altinkaynak, C.; Tavlasoglu, S.; Ozdemir, N.; Ocsoy, I. A new generation approach in enzyme immobilization: Organic–inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzym. Microb. Technol. 2016, 93–94, 105–112. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, Y.; Wang, J.; Huang, H.; Geng, X.; Tong, Y.; Wang, Z. Preparation of a Flower-Like Immobilized D-Psicose 3-Epimerase with Enhanced Catalytic Performance. Catalysts 2018, 8, 468. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Tian, R.; Xu, C.; Yung, B.C.; Wang, G.; Liu, Y.; Ni, Q.; Zhang, F.; Zhou, Z.; Wang, J.; et al. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nature Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Sun, J.; Ge, J.; Liu, W.; Lan, M.; Zhang, H.; Wang, P.; Wang, Y.; Niu, Z. Multi-enzyme co-embedded organic–inorganic hybrid nanoflowers: Synthesis and application as a colorimetric sensor. Nanoscale 2014, 6, 255–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Song, R.; Wang, M.; Yan, F.; He, L.; Feng, X.; Fang, S.; Zhao, J.; Zhang, H. Manganese(II) phosphate nanoflowers as electrochemical biosensorsfor the high-sensitivity detection of ractopamine. Sens. Actuators B Chem. 2015, 211, 310–317. [Google Scholar] [CrossRef]
- Omenetto, F.G.; Kaplan, D.L. New Opportunities for an Ancient Material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori Silk with Poly(ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Kook, G.; Jeong, S.; Kim, S.H.; Kim, M.K.; Lee, S.; Cho, I.-J.; Choi, N.; Lee, H.J. Wafer-Scale Multilayer Fabrication for Silk Fibroin-Based Microelectronics. ACS Appl. Mater. Interfaces 2019, 11, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Amsden, J.J.; Perry, H.; Boriskina, S.V.; Gopinath, A.; Kaplan, D.L.; Dal, L.N.; Omenetto, F.G. Spectral analysis of induced color change on periodically nanopatterned silk films. Opt. Express 2009, 17, 21271–21279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, S.; Li, C.; Miao, Y.; Zhu, L.; Mao, C.; Yang, M. Nucleation and Assembly of Silica into Protein-Based Nanocomposites as Effective Anticancer Drug Carriers Using Self-Assembled Silk Protein Nanostructures as Biotemplates. ACS Appl. Mater. Interfaces 2017, 9, 22259–22267. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Dai, F.; Ma, Y.; Yan, T.; Si, Y.; Sun, G. Ultrafine Silk-Derived Nanofibrous Membranes Exhibiting Effective Lysozyme Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 8777–8784. [Google Scholar] [CrossRef]
- Lei, Z.; Gao, C.; Chen, L.; He, Y.; Ma, W.; Lin, Z. Recent advances in biomolecule immobilization based on self-assembly: Organic–inorganic hybrid nanoflowers and metal-organic frameworks as novel substrates. J. Mater. Chem. B 2018, 6, 1581–1594. [Google Scholar] [CrossRef]
- Cooper, J.W.; Chen, J.; Li, Y.; Lee, C.S. Membrane-based nanoscale proteolytic reactor enabling protein digestion, peptide separation, and protein identification using mass spectrometry. Anal. Chem. 2003, 75, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Brenckle, M.A.; Partlow, B.; Tao, H.; Applegate, M.B.; Reeves, A.; Paquette, M.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Methods and Applications of Multilayer Silk Fibroin Laminates Based on Spatially Controlled Welding in Protein Films. Adv. Funct. Mater. 2016, 26, 44–50. [Google Scholar] [CrossRef]
- Brenckle, M.A.; Tao, H.; Kim, S.; Paquette, M.; Kaplan, D.L.; Omenetto, F.G. Protein-Protein Nanoimprinting of Silk Fibroin Films. Adv. Mater. 2013, 25, 2409–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Bao, N.; Prevelige, P.E.; Gupta, A. Fabrication of Ordered Nanostructures of Sulfide Nanocrystal Assemblies over Self-Assembled Genetically Engineered P22 Coat Protein. J. Am. Chem. Soc. 2010, 132, 17354–17357. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, Y.; Yin, Y.; Hu, W.; Liu, W.; Yang, H. Facile Synthesis of Enzyme-Inorganic Hybrid Nanoflowers and Its Application as a Colorimetric Platform for Visual Detection of Hydrogen Peroxide and Phenol. ACS Appl. Mater. Interfaces 2014, 6, 10775–10782. [Google Scholar] [CrossRef]
- Jianhua, H.; Chuanbao, C.; Faryal, I.; Xilan, M. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar]
- Zhang, Z.; Zhang, Y.; He, L.; Yang, Y.; Liu, S.; Wang, M.; Fang, S.; Fu, G. A feasible synthesis of Mn 3 (PO 4) 2 @BSA nanoflowers and its application as the support nanomaterial for Pt catalyst. J. Power Sources 2015, 284, 170–177. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhu, Y.J.; Zhao, J.; Lu, B.Q.; Chen, F.; Qi, C.; Wu, J. Hydroxyapatite nanosheet-assembled microspheres: Hemoglobin-templated synthesis and adsorption for heavy metal ions. J. Colloid. Interface Sci. 2014, 416, 11–18. [Google Scholar] [CrossRef]
- Aziz, S.; Sabzi, M.; Fattahi, A.; Arkan, E. Electrospun silk fibroin/PAN double-layer nanofibrous membranes containing polyaniline/TiO2 nanoparticles for anionic dye removal. J. Polym. Res. 2017, 24. [Google Scholar] [CrossRef]
- Gurbuz, O.; Okutan, M. Structural, electrical, and dielectric properties of Cr doped ZnO thin films: Role of Cr concentration. Appl. Surf. Sci. 2016, 387, 1211–1218. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genc, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roosta, M.; Ghaedi, M.; Shokri, N.; Daneshfar, A.; Sahraei, R.; Asghari, A. Optimization of the combined ultrasonic assisted/adsorption method for the removal of malachite green by gold nanoparticles loaded on activated carbon: Experimental design. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pappu, S.V.; Xu, Z. Detection of Local Density Distribution of Isolated Silanol Groups on Planar Silica Surfaces Using Nonlinear Optical Molecular Probes. Anal. Chem. 1998, 70, 4730–4735. [Google Scholar] [CrossRef]
- Tetsuo, A.; Akio, K.; Ryoko, T.; Saitô, H. Conformational characterization of Bombyx mori silk fibroin in the solid state by high-frequency carbon-13 cross polarization-magic angle spinning NMR, x-ray diffraction, and infrared spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar]











| Temperature Range | 30–127 °C | 127–361 °C | 361–648 °C |
|---|---|---|---|
| Cu3(PO4)2 | 7.32% | 2.57% | 8.67% |
| SF@Cu-NFs | 10.25% | 4.30% | 10.85% |
| SF protein | 19.71% | 49.63% | 14.48% |
| Model | Fitting Equation | Parameter | Value |
|---|---|---|---|
| Pseudo-first-order | y = −0.07x + 7.75 | (mg g−1) | 2312.00 |
| k1 (min−1) | 0.075 | ||
| 0.98 | |||
| Pseudo-second-order | y = 4 × 10−4x + 0.0023 | (mg g−1) | 2528.36 |
| k2 (g mg−1 min−1) | 6.92 × 10−5 | ||
| 0.99 | |||
| Intra-particle diffusion | y = 121.00x + 850.35 | C1 | 850.35 |
| 121.00 | |||
| 0.94 | |||
| y = 38.61x + 2022.19 | C2 | 2022.19 | |
| 38.61 | |||
| 0.70 | |||
| Result in this work | (mg g−1) | 2407.00 |
| Fitting Model | Langmuir Model y = 5.24 × 10−4x + 0.11 | Freundlich Model y = 0.1895x + 6.36 | Temkin Model y = 230.24x + 409.93 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | (mg g−1) | KL (mg L−1) | KF (mgn−1 g−1 L−n) | n | A | ||||
| Value | 1908.39 | 4.76 × 10−3 | 0.98 | 578.25 | 5.277 | 0.79 | 230.24 | 409.93 | 0.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xiong, Y.; Duan, M.; Wan, H.; Li, J.; Zhang, C.; Qin, S.; Fang, S.; Zhang, R. Investigation on the Adsorption-Interaction Mechanism of Pb(II) at Surface of Silk Fibroin Protein-Derived Hybrid Nanoflower Adsorbent. Materials 2020, 13, 1241. https://doi.org/10.3390/ma13051241
Li X, Xiong Y, Duan M, Wan H, Li J, Zhang C, Qin S, Fang S, Zhang R. Investigation on the Adsorption-Interaction Mechanism of Pb(II) at Surface of Silk Fibroin Protein-Derived Hybrid Nanoflower Adsorbent. Materials. 2020; 13(5):1241. https://doi.org/10.3390/ma13051241
Chicago/Turabian StyleLi, Xiang, Yan Xiong, Ming Duan, Haiqin Wan, Jun Li, Can Zhang, Sha Qin, Shenwen Fang, and Run Zhang. 2020. "Investigation on the Adsorption-Interaction Mechanism of Pb(II) at Surface of Silk Fibroin Protein-Derived Hybrid Nanoflower Adsorbent" Materials 13, no. 5: 1241. https://doi.org/10.3390/ma13051241