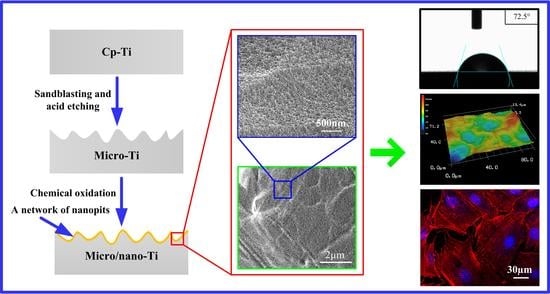
Construction of Complex Structures Containing Micro-Pits and Nano-Pits on the Surface of Titanium for Cytocompatibility Improvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Fabrication
2.2. Surface Characterization
2.3. Corrosion Resistance Evaluation
2.4. Cell Culture
2.5. Cell Attachment
2.6. Cell Morphology
2.7. Cell Proliferation
2.8. Alkaline Phosphatase (ALP) Activity Assay
2.9. Mineralization Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
3.2. Contact Angle Measurements
3.3. Corrosion-Resistance Measurement
3.4. Cell Attachment
3.5. Cell Morphology
3.6. Cell Proliferation
3.7. Cell Differentiation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Li, J.; He, X.; Hang, R.; Huang, X.; Tian, L.; Tang, B. Corrosion behavior of Zn-incorporated antibacterial TiO2 porous coating on titanium. Ceram. Int. 2016, 42, 17095–17100. [Google Scholar] [CrossRef]
- Wang, G.; Wan, Y.; Liu, Z. Incorporation of antibacterial ions on the micro/nanostructured surface and its effects on the corrosion behavior of titanium. Mater. Lett. 2018, 216, 303–305. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Song, R.; Huang, Q.; Yang, Y.; Lin, L.; Zhang, Y.; Jiang, P.; Duan, H.; Dong, X.; Lin, C. Electrochemical construction of a bio-inspired micro/nano-textured structure with cell-sized microhole arrays on biomedical titanium to enhance bioactivity. Electrochim. Acta 2015, 174, 1149–1159. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Su, Y.; Komasa, S.; Sekino, T.; Nishizaki, H.; Okazaki, J. Nanostructured Ti6Al4V alloy fabricated using modified alkali-heat treatment: Characterization and cell adhesion. Mater. Sci. Eng. C 2016, 59, 617–623. [Google Scholar] [CrossRef]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef]
- Shayganpour, A.; Rebaudi, A.; Cortella, P.; Diaspro, A.; Salerno, M. Electrochemical coating of dental implants with anodic porous titania for enhanced osteointegration. Beilstein J. Nanotechnol. 2015, 6, 2183–2192. [Google Scholar] [CrossRef]
- Cimenoglu, H.; Gunyuz, M.; Kose, G.T.; Baydogan, M.; Uğurlu, F.; Sener, C. Micro-arc oxidation of Ti6Al4V and Ti6Al7Nb alloys for biomedical applications. Mater. Character. 2011, 62, 304–311. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kwon, T.Y.; Kim, K.H. Surface modification of titanium and titanium alloys by ion implantation. J. Biomed. Mater. Res. B 2010, 93, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro-and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Lumetti, S.; Manfredi, E.; Ferraris, S.; Spriano, S.; Passeri, G.; Ghiacci, G.; Macaluso, G.; Galli, C. The response of osteoblastic MC3T3-E1 cells to micro-and nano-textured, hydrophilic and bioactive titanium surfaces. J. Mater. Sci. Mater. Med. 2016, 27, 68. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.; Lee, J.T.; Hong, J.S.; Lim, B.S.; Park, H.J.; Kim, Y.K.; Kim, T. Microgrooves on titanium surface affect peri-implant cell adhesion and soft tissue sealing; An in vitro and in vivo study. J. Periodontal. Implant Sci. 2015, 45, 120–126. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Lai, M.; Cai, K.; Hu, Y.; Yang, X.; Liu, Q. Regulation of the behaviors of mesenchymal stem cells by surface nanostructured titanium. Colloid Surf. B 2012, 97, 211–220. [Google Scholar] [CrossRef]
- Salerno, M.; Caneva-Soumetz, F.; Pastorino, L.; Patra, N.; Diaspro, A.; Ruggiero, C. Adhesion and proliferation of osteoblast-like cells on anodic porous alumina substrates with different morphology. IEEE Trans. Nanobiosci. 2013, 12, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.J.; ter Maat, J.; de Vos, W.M. Where bio meets nano: The many uses for nanoporous aluminum oxide in biotechnology. Biotechnol. Adv. 2012, 30, 1089–1099. [Google Scholar] [CrossRef]
- El Merhie, A.; Salerno, M.; Toccafondi, C.; Dante, S. Neuronal-like response of N2a living cells to nanoporous patterns of thin supported anodic alumina. Colloid Surf. B 2019, 178, 32–37. [Google Scholar] [CrossRef]
- Moon, B.S.; Kim, S.; Kim, H.E.; Jiang, T.S. Hierarchical micro-nano structured Ti6Al4V surface topography via two-step etching process for enhanced hydrophilicity and osteoblastic responses. Mater. Sci. Eng. Mater. Med. 2017, 73, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloid Surf. B 2016, 145, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, L.; Wu, Z.; Zhang, Y.; Chu, P.K. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials 2012, 33, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, K.; Lai, M.; Zhao, L.; Tang, L. Mesenchymal stem cells differentiation on hierarchically micro/nano—Structured titanium substrates. Adv. Eng. Mater. 2012, 14, B216–B223. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wan, Y.; Liu, Z. Fabrication of hierarchical micro/nanotopography on bio-titanium alloy surface for cytocompatibility improvement. J. Mater. Sci. 2016, 51, 9551–9561. [Google Scholar] [CrossRef]
- Wang, G.; Wan, Y.; Wang, T.; Liu, Z. Corrosion Behavior of Titanium Implant with Different Surface Morphologies. Procedia Manuf. 2017, 10, 363–370. [Google Scholar] [CrossRef]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, A.; Chiou, W.A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, G. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef] [PubMed]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-alloys and surface modifications to improve the mechanical properties and the biological response to orthopedic and dental implants: A review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Min, Y.; Liang, C.; Li, H.; Guo, L.; Liu, S.; Wang, H. Corrosion resistance and mechanical properties of titanium with hierarchical micro-nanostructure. Mater. Lett. 2016, 182, 43–46. [Google Scholar] [CrossRef]
- Zhang, R.; Ai, X.; Wan, Y.; Liu, Z.; Zhang, D.; Feng, S. Surface corrosion resistance in turning of titanium alloy. Int. J. Corros. 2015, 2015, 823172. [Google Scholar] [CrossRef]
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, Y.; Matsuno, T.; Hashimoto, Y.; Satoh, T. In vitro evaluation of H2O2 hydrothermal treatment of aged titanium surface to enhance biofunctional activity. Dent. Mater. J. 2013, 32, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Di Silvio, L.; Harper, E.J.; Bonfield, W. In vitro adhesion and biocompatibility of osteoblast-like cells to poly (methylmethacrylate) and poly (ethylmethacrylate) bone cements. J. Mater. Sci. Mater. Med. 2002, 13, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, V.; Shapiro, I.M.; Cavalcanti-Adam, E.A.; Composto, R.J.; Ducheyne, P.; Adams, C.S. Apoptosis and survival of osteoblast-like cells are regulated by surface attachment. J. Biol. Chem. 2005, 280, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Abron, A.; Hopfensperger, M.; Thompson, J.; Cooper, L.F. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J. Prosthet. Dent. 2001, 85, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Nishimura, I. Genes differentially expressed in titanium implant healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K.; Tuan, R.S. Regulation of human osteoblast integrin expression by orthopedic implant materials. Bone 1996, 18, 451–457. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Lim, J.Y.; Hansen, J.C.; Siedlecki, C.A.; Runt, J.; Donahue, H.J. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J. R. Soc. Interface 2005, 2, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti-Adam, E.A.; Volberg, T.; Micoulet, A.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007, 92, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bao, N.R.; Chen, S.; Zhao, J.N. Antimicrobial design of titanium surface that kill sessile bacteria but support stem cells adhesion. Appl. Surf. Sci. 2016, 389, 7–16. [Google Scholar] [CrossRef]
- Cooper, L.F. Biologic determinants of bone formation for osseointegration: Clues for future clinical improvements. J. Prosthet. Dent. 1998, 80, 439–449. [Google Scholar] [CrossRef]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef]
- Schneider, G.B.; Perinpanayagam, H.; Clegg, M.; Zaharias, R.; Seabold, D.; Keller, J.; Stanford, C. Implant surface roughness affects osteoblast gene expression. J. Dent. Res. 2003, 82, 372–376. [Google Scholar] [CrossRef]
- Dike, L.E.; Chen, C.S.; Mrksich, M.; Tien, J.; Whitesides, G.M.; Ingber, D.E. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. Vitro Cell. Dev. Biol. Anim. 1999, 35, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Liang, J.; Lin, C. Construction of micro–nano network structure on titanium surface for improving bioactivity. Appl. Surf. Sci. 2013, 280, 373–380. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H.; Kown, T.G.; Bae, Y.C.; Suh, J.Y. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater. 2010, 6, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cai, K.; Luo, Z.; Zhang, Y.; Li, L.; Lai, M.; Hou, Y.; Huang, Y.; Li, J.; Ding, X.; et al. Regulation of the differentiation of mesenchymal stem cells in vitro and osteogenesis in vivo by microenvironmental modification of titanium alloy surfaces. Biomaterials 2012, 33, 3515–3528. [Google Scholar]
- Wang, W.; Zhao, L.; Ma, Q.; Wang, Q.; Chu, P.K.; Zhang, Y. The role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials 2012, 33, 7993–8002. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.; Wu, K.; Ma, Q.; Mei, S.; Chu, P.K.; Wang, Q.; Zhang, Y. The role of integrin-linked kinase/β-catenin pathway in the enhanced MG63 differentiation by micro/nano-textured topography. Biomaterials 2013, 34, 631–640. [Google Scholar] [CrossRef] [PubMed]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wan, Y.; Liu, Z. Construction of Complex Structures Containing Micro-Pits and Nano-Pits on the Surface of Titanium for Cytocompatibility Improvement. Materials 2019, 12, 2820. https://doi.org/10.3390/ma12172820
Wang G, Wan Y, Liu Z. Construction of Complex Structures Containing Micro-Pits and Nano-Pits on the Surface of Titanium for Cytocompatibility Improvement. Materials. 2019; 12(17):2820. https://doi.org/10.3390/ma12172820
Chicago/Turabian StyleWang, Guisen, Yi Wan, and Zhanqiang Liu. 2019. "Construction of Complex Structures Containing Micro-Pits and Nano-Pits on the Surface of Titanium for Cytocompatibility Improvement" Materials 12, no. 17: 2820. https://doi.org/10.3390/ma12172820