Evaluation of Physicochemical Properties Composite Biodiesel from Waste Cooking Oil and Schleichera oleosa Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
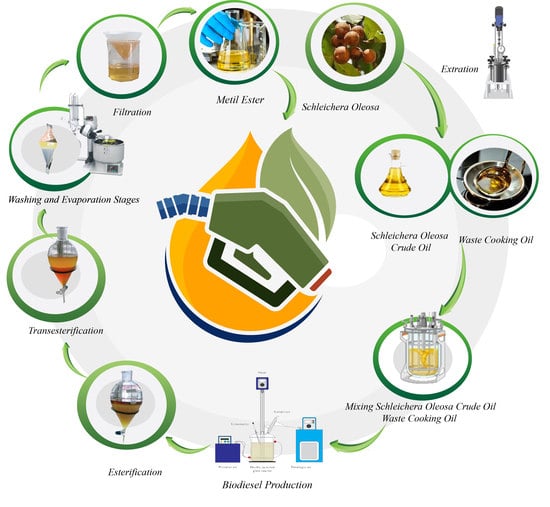
2.2. Equipment Set Up
2.3. Biodiesel Production
2.4. Analysis of Crude Oil and Biodiesel
2.5. DSC Measurement of WCME and WSME
3. Results and Discussion
3.1. Characterisation Properties of Crude Oil WCO, SO, and WCOK30
3.2. Density and Kinematic Viscosity of WCME, KOME, and WSME
3.3. Acid Value, the Concentration of Sulfur Residue, and Carbon Residue
3.4. Heating Value
3.5. Temperature
| Properties | Unit | Biodiesel | Diesel | WCME a | Waste Cooking [68] | KOME b | Kusum Oil [69] | WSME c | W70CI30 [7] | Improve |
|---|---|---|---|---|---|---|---|---|---|---|
| ASTM D6751 | ||||||||||
| Kinematic viscosity at 40 °C | mm2/s | 1.9–6.0 | 2.986 | 4.498 | 3.2 | 4.472 | 4.71 | 4.459 | 5.12 | −0.867 |
| Density at 40 °C | kg/m3 | 880 | 823 | 875 | 876 | 879 | 875.6 | 857 | 878 | −2.057 |
| Acid value | mg KOH/g | 0.5 (maks) | - | 0.132 | - | 0.308 | - | 0.28 | 0.57 | 112.12% |
| Higher heating value | MJ/kg | - | 46.200 | 40.047 | 38.431 | 39.889 | - | 39.952 | 40.82 | −0.237% |
| Oxidation stability at 110 °C | °h | 3 (min) | - | 3.3 | - | 9.03 | 3.2 | 6.8 | 18.14 | 106% |
| Flash Point | °C | 100–170 | 68.5 | 98 | 133 | 154 | 173 | 172 | 163.5 | 75.51% |
| Pour Point | °C | −15–16 | 6 | 12 | 3 | 9 | 4 | 9 | 4 | −25% |
| Cloud point | °C | −3–12 | 17 | 25 | 3 | 24 | 5 | 21 | 3 | −19.04% |
| Copper strip corrosion | -- | 3 (max) | 1a | 1a | - | 1a | 1a | 1a | 1a | - |
| Concentration sulfur residue | (w/w)% | - | 0.0760 | 0.0092 | - | 0.0093 | 3.2 | 0.0079 | 0.03 | −14.13% |
| Canradsons Carbon residue | (w/w)% | - | 0.00002 | 0.00015 | - | 0.00034 | - | 0.00034 | - | 844.44% |
3.6. Analysis Low-Temperature Properties of Methyl Ester
3.7. GaschromatographyMass Spectrometry (GC-MS) Analysis of Biodiesel
3.8. Oxidation Stability (OS) of Biodiesel
3.9. Effect of Catalyst Concentration
3.10. Effect of Reaction Times and Catalyst Concentration on Methyl Ester Yield
3.11. Effect of Methanol to Oil Molar Ratio on Methyl Ester Yield
3.12. FTIR Fourier-Transform Infrared (FT-IR) Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CP | Cloud Point |
| DSC | Differential Scanning Calorimetry |
| EN | European standard |
| FAME | Fatty acid Methyl Ester |
| FP | Flash Point |
| KOME | Schleichera oleosa methyl ester |
| OS | Oxidation Stability |
| PP | Pour Point |
| SO | Crude oil Schleichera oleosa oil |
| WCME | Waste cooking methyl ester |
| WCO | Crude Waste Cooking Oil |
| WCOK30 | Crude Waste Cooking Oil and Schleichera oleosa oil mixed |
| WSME | Waste Cooking Oil and Schleichera oleosa oil and mixed methyl ester |
References
- Vidigal, I.G.; Siqueira, A.F.; Melo, M.P.; Giordani, D.S.; da Silva, M.L.; Cavalcanti, E.H.; Ferreira, A.L. Applications of an electronic nose in the prediction of oxidative stability of stored biodiesel derived from soybean and waste cooking oil. Fuel 2021, 284, 119024. [Google Scholar] [CrossRef] [PubMed]
- Mortaza, A.; Soleiman, S.; Tabatabaei, M.; Soufiyan, M.M. Multi-objective exergetic and technical optimisation of a piezoelectric ultrasonic reactor applied to synthesise biodiesel from waste cooking oil (WCO) using soft computing techniques. Fuel 2019, 235, 100–112. [Google Scholar]
- Aitbelale, R.; Abala, I.; Alaoui, F.E.M.; Sahibed-Dine, A.; Rujas, N.M.; Aguilar, F. Characterization and determination of thermodynamic properties of waste cooking oil biodiesel: Experimental, correlation and modeling density over a wide temperature range up to 393.15 and pressure up to 140 MPa. Fluid Phase Equilibria 2019, 497, 87–96. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, J.; Gao, X.; Lin, P.; Zhang, Y.; Tan, P.; Lou, D. Waste cooking oil biodiesel and petroleum diesel soot from diesel bus: A comparison of morphology, nanostructure, functional group composition and oxidation reactivity. Fuel 2022, 321, 124019. [Google Scholar] [CrossRef]
- Sebayang, A.H.; Milano, J.; Alfansuri, M.; Silitonga, A.S.; Kusumo, F.; Prahmana, R.A.; Fayaz, H.; Zamri, M.F.M.A. Modelling and prediction approach for engine performance and exhaust emission based on artificial intelligence of sterculia foetida biodiesel. Energy Rep. 2022, 8, 8333–8345. [Google Scholar] [CrossRef]
- Helmi, M.; Tahvildari, K.; Hemmati, A.; Safekordi, A. Phosphomolybdic acid/graphene oxide as novel green catalyst using for biodiesel production from waste cooking oil via electrolysis method: Optimisation using with response surface methodology (RSM). Fuel 2021, 287, 119528. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.-H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimisation of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Lau, C.H.; Gan, S.; Lau, H.L.N.; Lee, L.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K. Insights into the effectiveness of synthetic and natural additives in improving biodiesel oxidation stability. Sustain. Energy Technol. Assess. 2022, 52, 102296. [Google Scholar] [CrossRef]
- Dharma, S.; Ong, H.C.; Masjuki, H.; Sebayang, A.; Silitonga, A. An overview of engine durability and compatibility using biodiesel–bioethanol–diesel blends in compression-ignition engines. Energy Convers. Manag. 2016, 128, 66–81. [Google Scholar] [CrossRef]
- Cordero-Ravelo, V.; Schallenberg-Rodriguez, J. Biodiesel production as a solution to waste cooking oil (WCO) disposal. Will any type of WCO do for a transesterification process? A quality assessment. J. Environ. Manag. 2018, 228, 117–129. [Google Scholar] [CrossRef]
- Guo, J.; Sun, S.; Liu, J. Conversion of waste frying palm oil into biodiesel using free lipase A from Candida antarctica as a novel catalyst. Fuel 2020, 267, 117323. [Google Scholar] [CrossRef]
- Mujtaba, M.; Masjuki, H.; Kalam, M.; Noor, F.; Farooq, M.; Ong, H.C.; Gul, M.; Soudagar, M.E.M.; Bashir, S.; Rizwanul Fattah, I. Effect of additivized biodiesel blends on diesel engine performance, emission, tribological characteristics, and lubricant tribology. Energies 2020, 13, 3375. [Google Scholar] [CrossRef]
- Ayetor, G.K.; Sunnu, A.; Parbey, J. Effect of biodiesel production parameters on viscosity and yield of methyl esters: Jatropha curcas, Elaeis guineensis and Cocos nucifera. Alex. Eng. J. 2015, 54, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Chinnamma, M.; Bhasker, S.; Madhav, H.; Devasia, R.M.; Shashidharan, A.; Pillai, B.C.; Thevannoor, P. Production of coconut methyl ester (CME) and glycerol from coconut (Cocos nucifera) oil and the functional feasibility of CME as biofuel in diesel engine. Fuel 2015, 140, 4–9. [Google Scholar] [CrossRef]
- Niyas, M.M.; Shaija, A. Performance evaluation of diesel engine using biodiesels from waste coconut, sunflower, and palm cooking oils, and their hybrids. Sustain. Energy Technol. Assess. 2022, 53, 102681. [Google Scholar]
- Colombo, K.; Santos, M.; Ender, L.; Barros, A.C. Production of biodiesel from soybean oil and methanol, catalysed by calcium oxide in a recycle reactor. S. Afr. J. Chem. Eng. 2019, 28, 19–25. [Google Scholar] [CrossRef]
- Elkelawy, M.; Bastawissi, H.A.-E.; Esmaeil, K.K.; Radwan, A.M.; Panchal, H.; Sadasivuni, K.K.; Suresh, M.; Israr, M. Maximization of biodiesel production from sunflower and soybean oils and prediction of diesel engine performance and emission characteristics through response surface methodology. Fuel 2020, 266, 117072. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Kusumo, F.; Mahlia, T.M.I.; Bahar, A.H. Pilot-scale production and the physicochemical properties of palm and Calophyllum inophyllum biodiesels and their blends. J. Clean. Prod. 2016, 126, 654–666. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Chong, W.T. Biodiesel conversion from high FFA crude jatropha curcas, calophyllum inophyllum and ceiba pentandra oil. Energy Procedia 2014, 61, 480–483. [Google Scholar] [CrossRef] [Green Version]
- Silitonga, A.S.; Mahlia, T.M.I.; Ong, H.C.; Riayatsyah, T.M.I.; Kusumo, F.; Husin, I.; Dharma, S.; Gumilang, D. A comparative study of biodiesel production methods for Reutealis trisperma biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 2006–2014. [Google Scholar] [CrossRef]
- Jamaluddin, N.A.M.; Riayatsyah, T.M.I.; Silitonga, A.S.; Mofijur, M.; Shamsuddin, A.H.; Ong, H.C.; Mahlia, T.M.I.; Rahman, S.M.A. Techno-economic analysis and physicochemical properties of Ceiba pentandra as second-generation biodiesel based on ASTM D6751 and EN 14214. Processes 2019, 7, 636. [Google Scholar] [CrossRef] [Green Version]
- Uyumaz, A. Combustion, performance and emission characteristics of a DI diesel engine fueled with mustard oil biodiesel fuel blends at different engine loads. Fuel 2018, 212, 256–267. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Cheah, M.Y.; Wang, C.-T. Physicochemical property enhancement of biodiesel synthesis from hybrid feedstocks of waste cooking vegetable oil and Beauty leaf oil through optimised alkaline-catalysed transesterification. Waste Manag. 2018, 80, 435–449. [Google Scholar] [CrossRef]
- Sudalaiyandi, K.; Alagar, K.; VJ, M.P.; Madhu, P. Performance and emission characteristics of diesel engine fueled with ternary blends of linseed and rubber seed oil biodiesel. Fuel 2021, 285, 119255. [Google Scholar]
- Alptekin, E.; Canakci, M.; Sanli, H. Biodiesel production from vegetable oil and waste animal fats in a pilot plant. Waste Manag. 2014, 34, 2146–2154. [Google Scholar] [CrossRef]
- Milano, J.; Shamsuddin, A.H.; Silitonga, A.; Sebayang, A.; Siregar, M.A.; Masjuki, H.; Pulungan, M.A.; Chia, S.R.; Zamri, M. Tribological study on the biodiesel produced from waste cooking oil, waste cooking oil blend with Calophyllum inophyllum and its diesel blends on lubricant oil. Energy Rep. 2022, 8, 1578–1590. [Google Scholar] [CrossRef]
- Ideris, F.; Zamri, M.F.M.A.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Fattah, I.M.R.; Mahlia, T.M.I. Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies 2022, 15, 7190. [Google Scholar] [CrossRef]
- Ong, H.C.; Tiong, Y.W.; Goh, B.H.H.; Gan, Y.Y.; Mofijur, M.; Fattah, I.M.R.; Chong, C.T.; Alam, M.A.; Lee, H.V.; Silitonga, A.S. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647. [Google Scholar] [CrossRef]
- Asl, M.A.; Tahvildari, K.; Bigdeli, T. Eco-friendly synthesis of biodiesel from WCO by using electrolysis technique with graphite electrodes. Fuel 2020, 270, 117582. [Google Scholar] [CrossRef]
- Isah, A.G.; Faruk, A.A.; Musa, U.G.; Mohammed, U.; Alhassan, M.; Abdullahi, U.B.; Damian, A.T. Oxidation stability and cold flow properties of biodiesel synthesised from castor oil: Influence of alkaline catalysts type and purification techniques. Mater. Today Proc. 2022, 57, 748–752. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; de Cinque Almeida, V.; da Silva, C. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.V.K.; Adil, S.F.; Assal, M.E.S.; Khan, M.R.; Warthan, M.A.; Siddiqui, A.; Rafiq, M.H. Production of biodiesel from waste cooking oil using ZnCuO/N-doped graphene nanocomposite as an efficient heterogeneous catalyst. Arab. J. Chem. 2021, 14, 102982. [Google Scholar] [CrossRef]
- Anilkumar, R.; Gupta, S.V.Y.; Virendra, K. Rathod. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Kurniati, S.; Soeparman, S.; Yuwono, S.S.; Hakim, L.; Syam, S. A Novel Process for Production of Calophyllum Inophyllum Biodiesel with Electromagnetic Induction. Energies 2019, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of biodiesel production using waste cooking oil and applying single and mixed immobilised lipases on polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, F.; Xu, L.; Peng, R.; Li, Y.; Deng, J. Batch and continuous esterification for the direct synthesis of high qualified biodiesel from waste cooking oils (WCO) with Amberlyst-15/Poly (vinylalcohol) membrane as a bifunctional catalyst. Chem. Eng. J. 2020, 388, 124214. [Google Scholar] [CrossRef]
- Pradhan, P.C.S.; Chakraborty, R. Optimization of infrared radiated fast and energy-efficient biodiesel production from waste mustard oil catalysed by Amberlyst 15: Engine performance and emission quality assessments. Fuel 2016, 173, 60–68. [Google Scholar] [CrossRef]
- Singh, D.S.D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Naveen, S.; Gopinath, K.P.; Malolan, R.; Ramesh, S.J.; Aakriti, K.; Arun, J. Novel Solar Parabolic Trough Collector cum Reactor for the Production of Biodiesel from Waste Cooking Oil using Calcium Oxide catalyst derived from seashells waste. Chem. Eng. Process. Process Intensif. 2020, 157, 108145. [Google Scholar] [CrossRef]
- Pölczmann, G.; Tóth, O.; Beck, Á.; Hancsók, J. Investigation of storage stability of diesel fuels containing biodiesel produced from waste cooking oil. J. Clean. Prod. 2016, 111, 85–92. [Google Scholar] [CrossRef]
- Wang, J.C.L.; Han, S. Effect of polymeric cold flow improvers on flow properties of biodiesel from waste cooking oil. Fuel 2014, 117, 876–881. [Google Scholar] [CrossRef]
- Bharti, R.B.S. Green tea (Camellia assamica) extract as an antioxidant additive to enhance the oxidation stability of biodiesel synthesised from waste cooking oil. Fuel 2020, 262, 116658. [Google Scholar] [CrossRef]
- Nanihar, N.K.A.; Hakim, F.; Sunar, N.M.; Manshoor, B.; Zaman, I. Influences of Storage Duration on the Fuel Properties of Biodiesel derived from Jatropha and Waste Cooking Oil. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1049, 012091. [Google Scholar] [CrossRef]
- Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Sulaiman, A.; Goli, S.A.H.; Tavassoli-Kafrani, E.; Ghaffari, A.; Rajaeifar, M.A.; Kim, K.-H.; Talebi, A.F.; et al. Unlocking the potential of walnut husk extract in the production of waste cooking oil-based biodiesel. Renew. Sustain. Energy Rev. 2020, 119, 109588. [Google Scholar] [CrossRef]
- Fu, J.; Turn, S.Q.; Takushi, B.M.; Kawamata, C.L. Storage and oxidation stabilities of biodiesel derived from waste cooking oil. Fuel 2016, 167, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Amran, N.A.; Bello, U.; Ruslan, M.S.H. The role of antioxidants in improving biodiesel’s oxidative stability, poor cold flow properties, and the effects of the duo on engine performance: A review. Heliyon 2022, 8, e09846. [Google Scholar] [CrossRef]
- Rajamohan, S.; Gopal, A.H.; Muralidharan, K.R.; Huang, Z.; Paramasivam, B.; Ayyasamy, T.; Nguyen, X.P.; Le, A.T.; Hoang, A.T. Evaluation of oxidation stability and engine behaviors operated by Prosopis juliflora biodiesel/diesel fuel blends with presence of synthetic antioxidant. Sustain. Energy Technol. Assess. 2022, 52, 102086. [Google Scholar] [CrossRef]
- Chrysikou, S.B.L.P. Oxidative stability of waste cooking oil and white diesel upon storage at room temperature. Bioresour. Technol. 2012, 126, 341–344. [Google Scholar] [CrossRef]
- Ideris, F.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Silitonga, A.S.; Ong, M.Y.; Ong, H.C.; Mahlia, T.M.I. Optimisation of ultrasound-assisted oil extraction from Canarium odontophyllum kernel as a novel biodiesel feedstock. J. Clean. Prod. 2021, 288, 125563. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Khurana, D.; Dhar, A. Improving oxidation stability of biodiesels derived from Karanja, Neem and Jatropha: Step forward in the direction of commercialisation. J. Clean. Prod. 2015, 107, 646–652. [Google Scholar] [CrossRef]
- Rashedul, H.; Masjuki, H.; Kalam, M.; Teoh, Y.; How, H.; Fattah, I.R. Effect of antioxidant on the oxidation stability and combustion–performance–emission characteristics of a diesel engine fueled with diesel–biodiesel blend. Energy Convers. Manag. 2015, 106, 849–858. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, W.; Ma, P.; Zhao, Z.; Xu, G.; Yang, C.; Chen, H.; Lin, H.; Han, S. Ternary blends of biodiesel with petro-diesel and diesel from direct coal liquefaction for improving the cold flow properties of waste cooking oil biodiesel. Fuel 2016, 177, 46–52. [Google Scholar] [CrossRef]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.-T.; Mahlia, T.M.I.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimisation and characterisation. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Dharma, S.; Hassan, M.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Milano, J. Experimental study and prediction of the performance and exhaust emissions of mixed Jatropha curcas-Ceiba pentandra biodiesel blends in diesel engine using artificial neural networks. J. Clean. Prod. 2017, 164, 618–633. [Google Scholar] [CrossRef]
- Kassem, Y.H.Ç. Effects of storage under different conditions on the fuel properties of biodiesel admixtures derived from waste frying and canola oils. Biomass Convers. Biorefinery 2018, 8, 825–845. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Wei, Z.; Dai, B.; Lin, H.; Han, S. Enhanced the Effects on Improving the Cold Flow Properties and Oxidative Stability of Diesel-Biodiesel Blends by Grafting Antioxidant on Pma Type Pour Point Depressant. Fuel Process. Technol. 2022, 238, 107483. [Google Scholar] [CrossRef]
- Jain, S.S.; Sharma, M.P. Effect of metal contents on oxidation stability of biodiesel/diesel blends. Fuel 2014, 116, 14–18. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Serqueira, D.S.; Pereira, J.F.; Squissato, A.L.; Rodrigues, M.A.; Lima, R.C.; Faria, A.M.; Richter, E.M.; Munoz, R.A. Oxidative stability and corrosivity of biodiesel produced from residual cooking oil exposed to copper and carbon steel under simulated storage conditions: Dual effect of antioxidants. Renew. Energy 2021, 164, 1485–1495. [Google Scholar] [CrossRef]
- Uğuz, G.; Atabani, A.; Mohammed, M.; Shobana, S.; Uğuz, S.; Kumar, G.; Al-Muhtaseb, A.H. Fuel stability of biodiesel from waste cooking oil: A comparative evaluation with various antioxidants using FT-IR and DSC techniques. Biocatal. Agric. Biotechnol. 2019, 21, 101283. [Google Scholar] [CrossRef]
- Devi, A.; Das, V.K.; Deka, D. A green approach for enhancing oxidation stability including long storage periods of biodiesel via Thuja oreantalis L. as an antioxidant additive. Fuel 2019, 253, 1264–1273. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, X.; Zhao, Y.; Chen, F.; Ren, F.; Lin, H.; Xue, Y.; Han, S. Bifunctional additive phenolic acids grafted ethylene-vinyl acetate copolymers for improving the cold flow properties and oxidative stability of waste cooking oil biodiesel-diesel blends. Fuel 2023, 332, 126005. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Sinha, A.; Das, B.; Dhakar, M.; Shinde, R.; Chakrabarti, A.; Yadav, V.; Bhatt, B. Kusum (Schleichera oleosa (Lour.) Oken): A potential multipurpose tree species, it’s future perspective and the way forward. Acta Ecol. Sin. 2022, 42, 565–571. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Shameer, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Rekhate, C.; Prajapati, A.K. Production, engine performance, combustion, emission characteristics and economic feasibility of biodiesel from waste cooking oil: A review. Environ. Qual. Manag. 2019, 29, 7–35. [Google Scholar] [CrossRef]
- Doğan, T.H. The testing of the effects of cooking conditions on the quality of biodiesel produced from waste cooking oils. Renew. Energy 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Damanik, N.; Ong, H.C.; Chong, W.; Silitonga, A. Biodiesel production from Calophyllum inophyllum−palm mixed oil. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1283–1289. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Wei, Z.; Dai, B.; Han, S. Synthesis and evaluation of bifunctional polymeric agent for improving cold flow properties and oxidation stability of diesel-biodiesel blends. Renew. Energy 2022, 196, 737–748. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. The effects of the fuel injection pressure on the performance and emission characteristics of a diesel engine fuelled with waste cooking oil biodiesel-diesel blends. Renew. Energy 2019, 132, 649–666. [Google Scholar] [CrossRef]
- Sia, C.B.; Kansedo, J.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Katre, G.; Raskar, S.; Zinjarde, S.; Kumar, V.R.; Kulkarni, B.; RaviKumar, A. Optimisation of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy 2018, 142, 944–952. [Google Scholar] [CrossRef]
- Atabani, A.; Shobana, S.; Mohammed, M.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Al-Muhtaseb, A.H. Integrated valorisation of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Silitonga, A.; Masjuki, H.; Ong, H.C.; Yusaf, T.; Kusumo, F.; Mahlia, T. Synthesis and optimisation of Hevea brasiliensis and Ricinus communis as feedstock for biodiesel production. Ind. Crops Prod. 2016, 85, 274–286. [Google Scholar] [CrossRef]
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Ong, H.C.; Mofijur, M.; Silitonga, A.; Gumilang, D.; Kusumo, F.; Mahlia, T. Physicochemical Properties of Biodiesel Synthesised from Grape Seed, Philippine Tung, Kesambi, and Palm Oils. Energies 2020, 13, 1319. [Google Scholar] [CrossRef] [Green Version]
- Silitonga, A.; Masjuki, H.; Mahlia, T.; Ong, H.C.; Kusumo, F.; Aditiya, H.; Ghazali, N. Schleichera oleosa L oil as feedstock for biodiesel production. Fuel 2015, 156, 63–70. [Google Scholar] [CrossRef]
- Aso, A.; Hassan, J.D.S. Investigation of microwave-assisted transesterification reactor of waste cooking oil. Renew. Energy 2020, 162, 1735–1746. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Production of biodiesel from waste cooking oil using a homogeneous catalyst: Study of semi-industrial pilot of microreactor. Renew. Energy 2019, 136, 677–682. [Google Scholar] [CrossRef]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR Analysis for Quantification of Fatty Acid Methyl Esters in Biodiesel Produced by Microwave-Assisted Transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964–969. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.H.; Abdullah, M.O.; Kansedo, J.; Mubarak, N.M.; Chan, Y.S.; Nolasco-Hipolito, C. Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones. Renew. Energy 2019, 139, 696–706. [Google Scholar] [CrossRef]
- Bai, H.; Tian, J.; Talifu, D.; Okitsu, K.; Abulizi, A. Process optimisation of esterification for deacidification in waste cooking oil: RSM approach and for biodiesel production assisted with ultrasonic and solvent. Fuel 2022, 318, 123697. [Google Scholar] [CrossRef]








| Type of Biodiesel | Improver | Loading | Cold Flow | Oxidation Stability (h) | Ref. | ||
|---|---|---|---|---|---|---|---|
| CP (°C) | PP (°C) | CFPP (°C) | |||||
| WCO | Polymethyl acrylate (PMA) | 0.04% | −9 | −17 | −11 | - | Wang [41] |
| WCO | WCO-Calophyllum inophyllum | 70:30 | 4 | 3 | 4 | 18.14 | Milano [7] |
| WCO | Green tea | 1000 ppm | - | - | - | 2.88 | Bharti [42] |
| WCO | Canola | Mixed oil (65:35) | 9 | 1.7 | - | 9.25 | Kassem [55] |
| WCO | Pyrogallol (PY) | 750 ppm | 9 | 11 | 3 | - | Uguz [60] |
| WCO | TBHQ | 1000 ppm | - | - | - | 51.2 | Zhou [55] |
| WCO | Thuja oreantalis L. | 100 ppm | - | - | - | 6.79 | Devi [61] |
| WCO | ethylene-vinyl acetate (EVA) | 0.1% | 5 | 10 | 11 | 25.56 | Sun [62] |
| Property | Unit | Crude Oils | Crude Oil Mixtures | ||||
|---|---|---|---|---|---|---|---|
| WCO a | WCO [23] | SO b | Kusum [63] | WCOK30 c | WC70JC30 [23] | ||
| Kinematic viscosity at 40 °C | mm2/s | 41.65 | 49.05 | 40.48 | 40.36 | 41.21 | 47.09 |
| Dynamic viscosity at 40 °C | mPa s | 37.65 | 44.27 | 36.36 | - | 37.05 | 42.52 |
| Density at 40 °C | kg/m3 | 916 | 902.70 | 915 | 860 | 916 | 902.90 |
| Acid value | mg KOH/g | 3.92 | 2.19 | 18 | - | 10 | 13.26 |
| Higher heating value | MJ/kg | 39.561 | 38.59 | 39.462 | 38.5 | 39.676 | 38.03 |
| Oxidation stability 110 °C | H | 3.5 | 3.6 | 9.3 | - | 7 | 1.38 |
| Property | Equipment | Standard Method |
|---|---|---|
| Kinematic viscosity at 40 °C | SVM 3000 viscometer cold properties (Anton Paar, Austria) | ASTM D455 |
| Density at 15 °C | SVM 3000 viscometer cold properties (Anton Paar, Austria) | ASTM D455 |
| Cloud and pour point | LAB 1300 ST Linetronic technologies, Switzerland | ASTM D2500 |
| Heating value | Automatic calorimeter, IKA C2000, China | ASTM D4809 |
| Acid number and iodine value | ECH 7000 Titrator Type TAN/TBN Titrator (ECH Germany) | ASTM D664 |
| Flash point | PMA 5 Pensky Martens flash point tester (Anton Paar, Austria) | ASTM D93 |
| Copper strip corrosion | KOEHLER k25339 Copper strip corrosion test tube bath | ASTM D130 |
| Sulfur content | Sulfur Meter X-Ray Tanaka Scientific RX 360SH | ASTM D4294 |
| Carbon residue | Koehler K80030 Condradson Carbon residue test | ASTM D189 |
| Oxidation stability at 110 °C | rapidOxy 100 fuel (oxidation stability tester (Anton Paar, Germany | ASTM 7525 |
| FAME content | Gas Chromatography system | EN 14103 |
| DSC | DSC 214 Polyma NETZSCH | ASTM D3418 |
| GC-MS | Shimadzu Type QP2010 Plus | ASTM6751 |
| FTIR | Bruker ALFA II | ASTM E2412 |
| Sample | Tonset (°C) | Tpeak-1 (°C) | Tpeak-2 (°C) | ΔH (J g−1) | Cp (J/g °C) | Peak Area J/g |
|---|---|---|---|---|---|---|
| WCME | 8.0 | 6.7 | −43.3 | −7.1 | −0.03 | −47.97 |
| WSME | 7.7 | 5.8 | −49.3 | −7.3 | 2.83 | −63.73 |
| FAME (w/w) % | Carbon Chain | WCME a | WCME [73] | KOME b | Kusum [63] | WSME c | W70CI30 [7] |
|---|---|---|---|---|---|---|---|
| Caprylic | C8:0 | - | 25 | - | - | - | - |
| Lauric | C12:0 | - | 3.2 | - | - | - | - |
| Myristic | C14:0 | - | 1.73 | 0.46 | 0.01 | 0.60 | 0.64 |
| Palmitic | C16:0 | 49.54 | 21.13 | 37.18 | 5–8 | 51.08 | 28.97 |
| Arachidic | C20:0 | - | - | - | - | - | - |
| Heneicosanoic | C21:0 | - | 1.79 | - | - | - | - |
| Stearic | C18:0 | 5.55 | 3.43 | - | 2–6 | 4.90 | 7.40 |
| Palmitoleic | C16:1 | - | 0.91 | - | - | - | |
| Heptadecanoic acid | C17:1 | - | 8.01 | - | - | - | - |
| Eicosanoic | C20:1 | - | 2 | - | |||
| Oleic | C18:1 | 38.16 | 21.01 | 45.56 | 2–3 | 36.25 | 44.15 |
| Linoleic | C18:2 | 11.77 | 7.54 | 5.56 | - | 14.11 | |
| Linolenic | C18:3 | 6.75 | - | - | 43–50 | 7.18 | 0.58 |
| Wavenumber (cm−1) | Group Attribution | Vibration Type | Absorption Intensity |
|---|---|---|---|
| 2922 | =C-H | Asymmetric stretching vibration | Strong |
| 2853 | -CH2 | Symmetric stretching vibration | Strong |
| 1741 | -C=O | Stretching | Strong |
| 1459 | -CH2 | Shear type vibration | Weak |
| 1439 | -CH3 | Shear type vibration | Middling |
| 1244 | -CH3 | Bending vibration | Weak |
| 1196 | C-O-C | Anti-symmetric stretching vibration | Middling |
| 1168 | C-O-C | Anti-symmetric stretching vibration | Middling |
| 721 | -CH2 | Plane rocking vibration | Weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suherman, S.; Abdullah, I.; Sabri, M.; Silitonga, A.S. Evaluation of Physicochemical Properties Composite Biodiesel from Waste Cooking Oil and Schleichera oleosa Oil. Energies 2023, 16, 5771. https://doi.org/10.3390/en16155771
Suherman S, Abdullah I, Sabri M, Silitonga AS. Evaluation of Physicochemical Properties Composite Biodiesel from Waste Cooking Oil and Schleichera oleosa Oil. Energies. 2023; 16(15):5771. https://doi.org/10.3390/en16155771
Chicago/Turabian StyleSuherman, Suherman, Ilmi Abdullah, Muhammad Sabri, and Arridina Susan Silitonga. 2023. "Evaluation of Physicochemical Properties Composite Biodiesel from Waste Cooking Oil and Schleichera oleosa Oil" Energies 16, no. 15: 5771. https://doi.org/10.3390/en16155771