Interfacial Thermal Conductance across Graphene/MoS2 van der Waals Heterostructures
Abstract
:1. Introduction
2. Simulation Methods
2.1. DFT Calculations
2.2. Molecular Dynamics Simulations
3. Result and Discussion
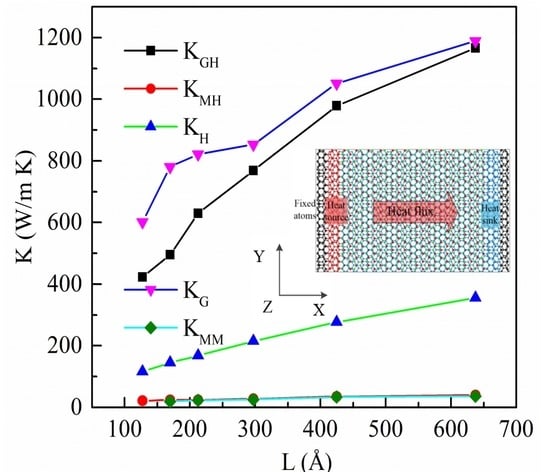
3.1. Thermal Conductivity of the Heterostructure
3.2. Thermal Conductance of Graphene/MoS2 Heterostructure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tries, A.; Osella, S.; Zhang, P.; Xu, F.; Ramanan, C.; Klaui, M.; Mai, Y.; Beljonne, D.; Wang, H.I. Experimental Observation of Strong Exciton Effects in Graphene Nanoribbons. Nano Lett. 2020, 20, 2993–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzerar, G.; Thebaud, S.; Pecorario, S.; Adessi, C. Drastic effects of vacancies on phonon lifetime and thermal conductivity in graphene. J. Phys. Condes. Matter 2020, 32, 295702. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Feng, S.Z.; Han, Y.; Gao, L.B.; Ly, T.H.; Xu, Z.P.; Lu, Y. Elastic straining of free-standing monolayer graphene. Nat. Commun. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Ruan, T.T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.L.; Dou, S.X. Graphene-based composites for electrochemical energy storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Oh, S.; Kim, C.K.; Kim, J. High Responsivity beta-Ga2O3 Metal-Semiconductor-Metal Solar-Blind Photodetectors with Ultraviolet Transparent Graphene Electrodes. ACS Photonics 2018, 5, 1123–1128. [Google Scholar] [CrossRef]
- Li, B.; Luo, J.C.; Huang, X.W.; Lin, L.W.; Wang, L.; Hu, M.J.; Tang, L.C.; Xue, H.G.; Gao, J.F.; Mai, Y.W. A highly stretchable, super-hydrophobic strain sensor based on polydopamine and graphene reinforced nanofiber composite for human motion monitoring. Compos. Pt. B Eng. 2020, 181, 107580. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C.N.R. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Ma, S.N.; Chiu, C.P.; Zhu, Y.J.; Tang, C.Y.; Long, H.; Qarony, W.; Zhao, X.H.; Zhang, X.M.; Lo, W.H.; Tsang, Y.H. Recycled waste black polyurethane sponges for solar vapor generation and distillation. Appl. Energy 2017, 206, 63–69. [Google Scholar] [CrossRef]
- Frisenda, R.; Navarro-Moratalla, E.; Gant, P.; De Lara, D.P.; Jarillo-Herrero, P.; Gorbachev, R.V.; Castellanos-Gomez, A. Recent progress in the assembly of nanodevices and van der Waals heterostructures by deterministic placement of 2D materials. Chem. Soc. Rev. 2018, 47, 53–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.W.; Huang, C.W.; Hu, S.B.; Deng, B.; Chen, Z.H.; Han, W.Q.; Chen, L. Negative and near-zero Poisson’s ratios in 2D graphene/MoS2 and graphene/h-BN heterostructures. J. Mater. Chem. C 2020, 8, 4021–4029. [Google Scholar] [CrossRef]
- Ren, K.; Sun, M.L.; Luo, Y.; Wan, S.K.; Yu, J.; Tang, W.C. First-principle study of electronic and optical properties of two-dimensional materials-based heterostructures based on transition metal dichalcogenides and boron phosphide. Appl. Surf. Sci. 2019, 476, 70–75. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.D.; Wu, G.J.; Wang, Z.; Fang, H.H.; Lin, T.; Sun, S.; Shen, H.; Hu, W.D.; Wang, J.L.; et al. High-Performance Photovoltaic Detector Based on MoTe2/MoS2 Van der Waals Heterostructure. Small 2018, 14, 1703293. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.Z.; Xu, W.Y.; Pan, J.B.; Song, S.R.; Zhang, Y.; Zhou, H.T.; Wang, Y.L.; Bao, L.H.; Zhang, Y.Y.; et al. Stable Silicene in Graphene/Silicene Van der Waals Heterostructures. Adv. Mater. 2018, 30, 1804650. [Google Scholar] [CrossRef]
- Biroju, R.K.; Das, D.; Sharma, R.; Pal, S.; Mawlong, L.P.L.; Bhorkar, K.; Giri, P.K.; Singh, A.K.; Narayanan, T.N. Hydrogen Evolution Reaction Activity of Graphene-MoS2 van der Waals Heterostructures. ACS Energy Lett. 2017, 2, 1355–1361. [Google Scholar] [CrossRef]
- Safeer, C.K.; Ingla-Aynes, J.; Herling, F.; Garcia, J.H.; Vila, M.; Ontoso, N.; Calvo, M.R.; Roche, S.; Hueso, L.E.; Casanova, F. Room-Temperature Spin Hall Effect in Graphene/MoS2 van der Waals Heterostructures. Nano Lett. 2019, 19, 1074–1082. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.L.; Zhang, B.T.; Li, Y.L.; Luo, X.Y.; Li, G.R.; Chen, Y.X.; Zhang, C.Q.; He, J.L. Tunable Ultrafast Nonlinear Optical Properties of Graphene/MoS2 van der Waals Heterostructures and Their Application in Solid-State Bulk Lasers. ACS Nano 2018, 12, 11376–11385. [Google Scholar] [CrossRef]
- Gong, F.; Ding, Z.W.; Fang, Y.; Tong, C.J.; Xia, D.W.; Lv, Y.Y.; Wang, B.; Papavassiliou, D.V.; Liao, J.X.; Wu, M.Q. Enhanced Electrochemical and Thermal Transport Properties of Graphene/MoS2 Heterostructures for Energy Storage: Insights from Multiscale Modeling. ACS Appl. Mater. Interfaces 2018, 10, 14614–14621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Xie, Y.E.; Peng, Q.; Chen, Y.P. Thermal transport in MoS2/Graphene hybrid nanosheets. Nanotechnology 2015, 26, 375402. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Kim, Y.; Chung, S.; Kim, H.; Son, J.G. Fabrication of a MoS2/Graphene Nanoribbon Heterojunction Network for Improved Thermoelectric Properties. Adv. Mater. Interfaces 2019, 6, 1901333. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.D.; Dai, Y.; Guo, M.; Niu, C.W.; Huang, B.B. Graphene adhesion on MoS2 monolayer: An ab initio study. Nanoscale 2011, 3, 3883–3887. [Google Scholar] [CrossRef]
- Qiu, B.; Zhao, X.W.; Hu, G.C.; Yue, W.W.; Ren, J.F.; Yuan, X.B. Optical Properties of Graphene/MoS2 Heterostructure: First Principles Calculations. Nanomaterials 2018, 8, 962. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.W.; Pei, Q.X.; Jiang, J.W.; Huang, W.X.; Zhang, Y.W. Interfacial thermal conductance in graphene/MoS2 heterostructures. Carbon 2016, 96, 888–896. [Google Scholar] [CrossRef]
- Nakamura, H.; Mohammed, A.; Rosenzweig, P.; Matsuda, K.; Nowakowski, K.; Kuster, K.; Wochner, P.; Ibrahimkutty, S.; Wedig, U.; Hussain, H.; et al. Spin splitting and strain in epitaxial monolayer WSe2 on graphene. Phys. Rev. B 2020, 101, 8. [Google Scholar] [CrossRef] [Green Version]
- Naumov, N.G.; Korlyukov, A.A.; Piryazev, D.A.; Virovets, A.V.; Fedorov, V.E. High-precision X-ray diffraction data, experimental and theoretical study of 2H-MoS2. Russ. Chem. Bull. 2013, 62, 1852–1857. [Google Scholar] [CrossRef]
- Tornatzky, H.; Gillen, R.; Uchiyama, H.; Maultzsch, J. Phonon dispersion in MoS. Phys. Rev. B 2019, 99, 144309. [Google Scholar] [CrossRef] [Green Version]
- Schumann, T.; Dubslaff, M.; Oliveira, M.H.; Hanke, M.; Lopes, J.M.J.; Riechert, H. Effect of buffer layer coupling on the lattice parameter of epitaxial graphene on SiC(0001). Phys. Rev. B 2014, 90, 5. [Google Scholar] [CrossRef] [Green Version]
- Phuc, H.V.; Hieu, N.N.; Hoi, B.D.; Phuong, L.T.T.; Nguyen, C.V. First principle study on the electronic properties and Schottky contact of graphene adsorbed on MoS2 monolayer under applied out-plane strain. Surf. Sci. 2018, 668, 23–28. [Google Scholar] [CrossRef]
- Jin, C.J.; Rasmussen, F.A.; Thygesen, K.S. Tuning the Schottky Barrier at the Graphene/MoS2 Interface by Electron Doping: Density Functional Theory and Many-Body Calculations. J. Phys. Chem. C 2015, 119, 19928–19933. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, L.; Broido, D.J.P.R.B. Optimized Tersoff and Brenner empirical potential parameters for lattice dynamics and phonon thermal transport in carbon nanotubes and graphene. Phys. Rev. B 2010, 81, 205441. [Google Scholar] [CrossRef] [Green Version]
- Stillinger, W. Computer simulation of local order in condensed phases of silicon. Phys. Rev. B Condens. Matter 1985, 31, 5262–5271. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.W. Parametrization of Stillinger-Weber potential based on valence force field model: Application to single-layer MoS2 and black phosphorus. Nanotechnology 2015, 26, 315706. [Google Scholar] [CrossRef] [Green Version]
- Vazirisereshk, M.R.; Ye, H.; Ye, Z.J.; Otero-de-la-Roza, A.; Zhao, M.Q.; Gao, Z.L.; Johnson, A.T.C.; Johnson, E.R.; Carpick, R.W.; Martini, A. Origin of Nanoscale Friction Contrast between Supported Graphene, MoS2, and a Graphene/MoS2 Heterostructure. Nano Lett. 2019, 19, 5496–5505. [Google Scholar] [CrossRef]
- Liu, B.; Baimova, J.A.; Reddy, C.D.; Law, A.W.K.; Dmitriev, S.V.; Wu, H.; Zhou, K. Interfacial Thermal Conductance of a Silicene/Graphene Bilayer Heterostructure and the Effect of Hydrogenation. ACS Appl. Mater. Interfaces 2014, 6, 18180–18188. [Google Scholar] [CrossRef]
- Yan, R.S.; Simpson, J.R.; Bertolazzi, S.; Brivio, J.; Watson, M.; Wu, X.F.; Kis, A.; Luo, T.F.; Walker, A.R.H.; Xing, H.G. Thermal Conductivity of Monolayer Molybdenum Disulfide Obtained from Temperature-Dependent Raman Spectroscopy. ACS Nano 2014, 8, 986–993. [Google Scholar] [CrossRef]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Srinivasan, S.; Balasubramanian, G. Reduced Thermal Transport in the Graphene/MoS2/Graphene Heterostructure: A Comparison with Freestanding Monolayers. Langmuir 2018, 34, 3326–3335. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.Y.; Cai, K.; Jiang, J.W.; Zheng, J.C.; Zhao, J.H.; Wei, N. Interfacial thermal conductance in graphene/black phosphorus heterogeneous structures. Carbon 2017, 117, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ong, Z.Y.; Wu, J.; Zhao, Y.S.; Watanabe, K.; Taniguchi, T.; Chi, D.Z.; Zhang, G.; Thong, J.T.L.; Qiu, C.W.; et al. Thermal Conductance of the 2D MoS2/h-BN and graphene/h-BN Interfaces. Sci Rep. 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.F.; Minhaj, T.; Huang, L.J.; Yu, Y.L.; Cao, L.Y. In-Plane and Interfacial Thermal Conduction of Two-Dimensional Transition-Metal Dichalcogenides. Phys. Rev. Appl. 2020, 13, 034059. [Google Scholar] [CrossRef]
- Rahman, M.; Shahzadeh, M.; Pisana, S. Simultaneous measurement of anisotropic thermal conductivity and thermal boundary conductance of 2-dimensional materials. J. Appl. Phys. 2019, 126, 205103. [Google Scholar] [CrossRef]
- Wu, Y.J.; Lan, Y.W.; Hsu, S.C.; Yeh, C.H.; Ku, Y.S.; Jiang, J.C.; Xu, Y.B. Tuning Interfacial Thermal and Electrical Conductance across a Metal/MoS2 Monolayer through N-Methyl-2-pyrrolidone Wet Cleaning. Adv. Mater. Interfaces 2020, 2000364. [Google Scholar] [CrossRef]
- Aiyiti, A.; Bai, X.; Wu, J.; Xu, X.F.; Li, B.W. Measuring the thermal conductivity and interfacial thermal resistance of suspended MoS2 using electron beam self-heating technique. Sci. Bull. 2018, 63, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.Z.; Zhao, C.Y.; Gu, X.K. Thermal conductivity of MoS2/MoSe2 heterostructures: The role of lattice mismatch, interlayer rotation and species intermixing. Int. J. Heat Mass Transf. 2019, 143, 118583. [Google Scholar] [CrossRef]
- Hong, Y.; Ju, M.G.; Zhang, J.C.; Zeng, X.C. Phonon thermal transport in a graphene/MoSe2 van der Waals heterobilayer. Phys. Chem. Chem. Phys. 2018, 20, 2637–2645. [Google Scholar] [CrossRef]
- Liu, B.; Meng, F.M.; Reddy, C.D.; Baimova, J.A.; Srikanth, N.; Dmitriev, S.V.; Zhou, K. Thermal transport in a graphene-MoS2 bilayer heterostructure: A molecular dynamics study. RSC Adv. 2015, 5, 29193–29200. [Google Scholar] [CrossRef]
- Casimir, H.B.G. Note on the Conduction of Heat in Crystals. Physica 1938, 5, 495–500. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.X.; Rabczuk, T. Thermal conductivity of single-layer MoS2: A comparative study between 1H and 1T′ phases. Physica E 2018, 103, 294–299. [Google Scholar] [CrossRef]
- Cao, A.J.; Qu, J.M. Kapitza conductance of symmetric tilt grain boundaries in graphene. J. Appl. Phys. 2012, 111, 053529. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Haskins, J.; Kinaci, A.; Sevik, C.; Sevincli, H.; Cuniberti, G.; Cagin, T. Control of Thermal and Electronic Transport in Defect-Engineered Graphene Nanoribbons. ACS Nano 2011, 5, 3779–3787. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.Z.; Li, Y.L.; Lee, G.H.; Cui, X.; Chenet, D.; You, Y.M.; Heinz, T.F.; Hone, J.C. Measurement of Lateral and Interfacial Thermal Conductivity of Single- and Bilayer MoS2 and MoSe2 Using Refined Optothermal Raman Technique. ACS Appl. Mater. Interfaces 2015, 7, 25923–25929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, J.D.; Rohl, A.L. The General Utility Lattice Program (GULP). Mol. Simul. 2003, 29, 291–341. [Google Scholar] [CrossRef]









| Ref. | Materials | Method | Lattice Constant a (Å) |
|---|---|---|---|
| Ma et al. [23] | Monolayer graphene | First-principles | a = 2.45 |
| Monolayer MoS2 | a = 3.12 | ||
| Qin et al. [24] | Monolayer graphene | First-principles | a = 2.47 |
| Monolayer MoS2 | a = 3.16 | ||
| Ding et al. [25] | Monolayer graphene | First-principles | a = 2.49 |
| Monolayer MoS2 | a = 3.12 | ||
| Nakamura [26] | Graphene beneath WSe2 | Experiment | a = 2.4575 ± 0.0007 |
| Tornatzky [27] | Multilayer MoS2 | Experiment | a = 3.161 |
| Bilayer MoS2 | First-principles | a = 3.158 | |
| Naumov [28] | Multilayer MoS2 | Experiment | a = 3.16 |
| Schumann [29] | Bilayer graphene | Experiment | a = 2.467 |
| Monolayer graphene/Bilayer graphene | a = 2.456/2.463 | ||
| This work | Monolayer graphene | First-principles | a = 2.45/2.47 |
| Monolayer MoS2 | a = 3.12/3.16 |
| Ref. | Materials | Method | Temperature (K) | K (W/m K) | G (MW/m2 K) |
|---|---|---|---|---|---|
| Srinivasan et al. [40] | graphene/MoS2/graphene | MD | 300 | 17–65 | 0.76–0.9 |
| Chen et al. [41] | BP/graphene | MD | 100–350 | 25.33–56.34 | |
| Liu et al. [42] | MoS2/h-BN graphene/h-BN | Experiment | Room temperature | 17 ± 0.4 52.2 ± 2.1 | |
| Yu et al. [43] | MoS2/SiO2 | Experiment | Room temperature | 32.5 ± 3.4 | 18.6 |
| Rahuman et al. [44] | graphene/SiO2 MoS2/SiO2 | Experiment | Room temperature | 636 ± 140 63 ± 22 | |
| Wu et al. [45] | Au/MoS2 | Experiment | Room temperature | 5.12 | |
| Aiyiti et al. [46] | MoS2/pt | Experiment | Room temperature | 30 | 0.5 |
| Zheng et al. [47] | MoS2/MoSe2 | MD | 300 | 17.48 | |
| Hong et al. [48] | graphene/MoSe2 | MD | 300 | 138.24 | 5.26 |
| Liu et al. [49] | graphene/MoS2 | MD | 350 | 31.74 | 5.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wang, J.; Xie, H.; Guo, Z. Interfacial Thermal Conductance across Graphene/MoS2 van der Waals Heterostructures. Energies 2020, 13, 5851. https://doi.org/10.3390/en13215851
Wu S, Wang J, Xie H, Guo Z. Interfacial Thermal Conductance across Graphene/MoS2 van der Waals Heterostructures. Energies. 2020; 13(21):5851. https://doi.org/10.3390/en13215851
Chicago/Turabian StyleWu, Shuang, Jifen Wang, Huaqing Xie, and Zhixiong Guo. 2020. "Interfacial Thermal Conductance across Graphene/MoS2 van der Waals Heterostructures" Energies 13, no. 21: 5851. https://doi.org/10.3390/en13215851