Seasonal Energy Flexibility Through Integration of Liquid Sorption Storage in Buildings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Building
2.2. System Description
2.3. Liquid Sorption Storage Modelling
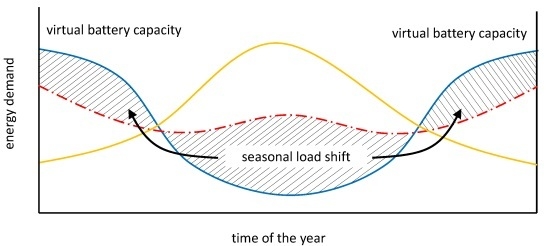
2.4. Seasonal Flexibility Analysis
3. Results
3.1. Coefficient of Performance (COP) Improvement and Electric Load Shifting, i.e., Seasonal Energy Flexibilities
3.2. Storage Capacity and Size
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Eflex,charging | Negative energy flexibility offered during charging (kWh) |
| Eflex,discharging | Positive energy flexibility offered during discharging (kWh) |
| Pel,hp,charging | Electric power of heat pump during charging (kW) |
| Pel,storage,discharging | Electric power of heat pump during discharging (kW) |
| Pel,ref,heating | Electric power of heat pump without storage during heating (kW) |
| Δtexcess | Time of excess electricity available (hours) |
| Δtheating | Time with space heating demand (hours) |
| ηcharging | Charging efficiency (%) |
| ηisen | Isentropic efficiency of heat pump (%) |
| Qhp,desorption | Heat provided by heat pump during charging of storage (kWh) |
| Qheating | Space heating demand (kWh) |
| COPhp,charging | Heat pump COP during charging |
| COPstorage,discharging | Heat pump COP during discharging |
| COPref,heating | Heat pump COP without storage during heating |
| Tdesorption | Sorbent temperature in HMX desorber during charging (°C) |
| Tsat,p_desorb | Saturation temperature of water at desorption pressure (°C) |
| Tsupply | Supply temperature of space heating system (°C) |
| Tamb | Ambient air temperature (°C) |
| THMX,ev,in | Inlet temperature at HMX evaporator (°C) |
| ΔThx,HMX | Temp. difference primary/secondary fluid in HMX heat exchanger (K) |
| ΔThx,hp | Temperature difference primary/secondary fluid in hp heat exchanger (K) |
| ΔTHMX,ev | Temperature difference of HTF across HMX evaporator/condenser (K) |
| ΔTair | Temperature difference ambient air inlet to evaporator water outlet (K) |
| ΔT50,max | Maximum temperature lift sorption storage (K) |
| Msorbent,in | Concentrated sorbent mass entering HMX absorber in discharge (kg) |
| Msorbent,out | Diluted sorbent mass leaving HMX absorber in discharge (kg) |
| hsorbent,in | Specific enthalpy of sorbent entering HMX absorber (kJ/(kg·K)) |
| hsorbent,out | Specific enthalpy of sorbent leaving HMX absorber (kJ/(kg·K)) |
| hlg | Specific enthalpy of condensation for water (kJ/(kg·K)) |
| csorbent,in | NaOH concentration of sorbent entering HMX absorber (kg/kg) |
| csorbent,out | NaOH concentration of sorbent leaving HMX absorber (kg/kg) |
Abbreviations
| COP | Coefficient of performence |
| HMX | Heat and mass exchanger |
| hp | Heat pump |
| H2O | Water |
| NaOH | Sodium hydroxide |
| PV | Photovoltaics |
| SFH | Single family home |
| SFH45 | Single family home with specific space heating demand of 45 kWh/m2 |
References
- Rathgeber, C.; Hiebler, S.; Lävemann, E.; Pablo, D.; Ana, L.; Jaume, G.; Alvaro de, G.C.; Laia, M.; Luisa, F.C.; Andreas, K.-H.; et al. IEA SHC Task 42 / ECES Annex 29—A Simple Tool for the Economic Evaluation of Thermal Energy Storages. Energy Procedia 2016, 91, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Zondag, H. Chapter 6–Sorption Heat Storage. In Solar Energy Storage; Sørensen, B., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 135–154. [Google Scholar]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Riffat, S. Thermochemical energy storage technologies for building applications: A state-of-the-art review. Int. J. Low Carbon Technol. 2012, 8, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Bales, C. Chemical and sorption heat storage. In Proceedings of the DANVAK Seminar, Lyngby, Denmark, 14 November 2006. [Google Scholar]
- Hadorn, J.C. Thermal Energy Storage for Solar and Low Energy Buildings: State of the Art; Universidad de Lleida: Lleida, Spain, 2005. [Google Scholar]
- Fumey, B.; Weber, R.; Baldini, L. Liquid sorption heat storage—A proof of concept based on lab measurements with a novel spiral fined heat and mass exchanger design. Appl. Energy 2017, 200, 215–225. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Dawoud, B. Unified Methodology to Identify the Potential Application of Seasonal Sorption Storage Technology. Energies 2020, 13, 1037. [Google Scholar] [CrossRef] [Green Version]
- Fumey, B.; Weber, R.; Baldini, L. Sorption based long-term thermal energy storage—Process classification and analysis of performance limitations: A review. Renew. Sustain. Energy Rev. 2019, 111, 57–74. [Google Scholar] [CrossRef]
- Heier, J.; Bales, C.; Martin, V. Combining thermal energy storage with buildings—A review. Renew. Sustain. Energy Rev. 2015, 42, 1305–1325. [Google Scholar] [CrossRef]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas—Solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Krese, G.; Koželj, R.; Butala, V.; Stritih, U. Thermochemical seasonal solar energy storage for heating and cooling of buildings. Energy Build. 2018, 164, 239–253. [Google Scholar] [CrossRef]
- Fumey, B.; Weber, R.; Gantenbein, P.; Daguenet-Frick, X.; Williamson, T.; Dorer, V. Development of a Closed Sorption Heat Storage Prototype. Energy Procedia 2014, 46, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Aydin, D.; Casey, S.P.; Chen, X.; Riffat, S. Numerical and experimental analysis of a novel heat pump driven sorption storage heater. Appl. Energy 2018, 211, 954–974. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; You, T.; Wang, J.; Wang, B.; Shi, W.; Li, X. A novel internally hybrid absorption-compression heat pump for performance improvement. Energy Convers. Manag. 2018, 168, 237–251. [Google Scholar] [CrossRef]
- Palomba, V.; Dino, G.E.; Frazzica, A. Coupling sorption and compression chillers in hybrid cascade layout for efficient exploitation of renewables: Sizing, design and optimization. Renew. Energy 2020, 154, 11–28. [Google Scholar] [CrossRef]
- Dott, R.; Haller, M.Y.; Ruschenburg, J.; Ochs, J.; Bony, J. The Reference Framework for System Simulations of the IEA SHC Task 44/HPP Annex 38 Part B: Buildings and Space Heat Load. Available online: https://task44.iea-shc.org/publications (accessed on 27 April 2020).
- Climate Strasbourg—Monthly Temperatures. Available online: https://de.climate-data.org (accessed on 20 April 2020).
- Fumey, B.; Weber, R.; Gantenbein, P.; Daguenet-Frick, X.; Williamson, T.; Dorer, V. Closed Sorption Heat Storage based on Aqueous Sodium Hydroxide. Energy Procedia 2014, 48, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Köll, R.; van Helden, W.; Fumey, B. European Union Seventh Framework Program Project COMTES—Combined Development of Compact Thermal Energy Storage Technologies—Deliverable 5.1: Description of Experimental Systems. Johansen, J.B., Furbo, S., Eds.; Available online: https://cordis.europa.eu/project/id/295568 (accessed on 27 April 2020).
- Olsson, J.; Jernqvist, Å.; Aly, G. Thermophysical properties of aqueous NaOH−H2O solutions at high concentrations. Int. J. Thermophys. 1997, 18, 779–793. [Google Scholar] [CrossRef]
- Gasser, L.; Flück, S.; Kleingries, M.; Meier, C.; Bätschmann, M.; Wellig, B. High efficiency heat pumps for low temperature lift applications. In Proceedings of the 12th IEA Heat Pump Conference, Rotterdam, The Netherlands, 12–14 May 2017. [Google Scholar]
- Storage Lakes for a Sucessful Energy Transition (translated). Factsheet 2019; Swiss Association of Hydropower Economy. Available online: https://www.swv.ch/fachinformationen/wasserkraft-schweiz/ (accessed on 27 April 2020).






| Input Variables | Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul | Aug. | Sep. | Oct. | Nov. | Dec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH load (kWh/m2) | 11.99 | 8.18 | 4.53 | 0.96 | 0.025 | 0 | 0 | 0 | 0 | 1.55 | 7.62 | 11.4 |
| Tsup (°C) | 30.1 | 29.1 | 27.4 | 26.1 | 27.2 | 0 | 0 | 0 | 0 | 26.4 | 28.1 | 29.4 |
| Tret (°C) | 22.2 | 21.4 | 20.7 | 20.4 | 20.5 | 0 | 0 | 0 | 0 | 20.5 | 21 | 22 |
| Tamb (°C) | 0.9 | 2.4 | 6.1 | 9.7 | 13.8 | 17.2 | 19.2 | 18.6 | 15.7 | 10.7 | 5.3 | 2.1 |
| Input Parameters | |||||
|---|---|---|---|---|---|
| ηisen | 0.5 | csorbent,in | 0.5 | ΔThx,HMX (K) | 3 |
| ηcharging | 0.9 | Tdesorption (°C) | 55 | ΔThx,hp (K) | 3 |
| Δtexcess (h) | 720 | nominal heat pump/HMX capacity (kW) | 4.07 | ΔTHMX,ev (K) | 3 |
| ΔTair (K) | 10 | ΔT50,max (K) | 25 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, L.; Fumey, B. Seasonal Energy Flexibility Through Integration of Liquid Sorption Storage in Buildings. Energies 2020, 13, 2944. https://doi.org/10.3390/en13112944
Baldini L, Fumey B. Seasonal Energy Flexibility Through Integration of Liquid Sorption Storage in Buildings. Energies. 2020; 13(11):2944. https://doi.org/10.3390/en13112944
Chicago/Turabian StyleBaldini, Luca, and Benjamin Fumey. 2020. "Seasonal Energy Flexibility Through Integration of Liquid Sorption Storage in Buildings" Energies 13, no. 11: 2944. https://doi.org/10.3390/en13112944