Improved Solubility Model for Pure Gas and Binary Mixture of CO2-H2S in Water: A Geothermal Case Study with Total Reinjection
Abstract
:1. Introduction
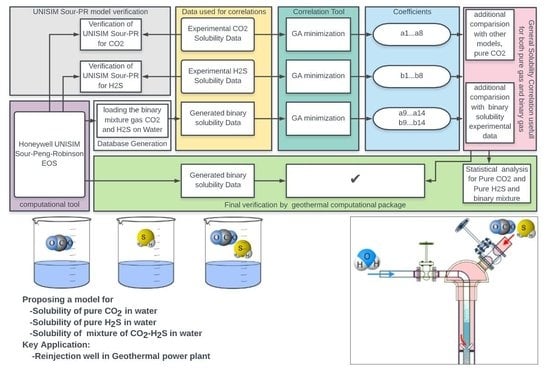
2. Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tester, J.; Reber, T.; Beckers, K.; Lukawski, M.; Camp, E.; Aguirre, G.A.; Horowitz, F. Integrating Geothermal Energy Use Into Re-Building American Infrastructure. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 19–25 April 2015. [Google Scholar]
- Manente, G.; Lazzaretto, A.; Bardi, A.; Paci, M. Geothermal power plant layouts with water absorption and reinjection of H2S and CO2 in fields with a high content of non-condensable gases. Geothermics 2019, 78, 70–84. [Google Scholar] [CrossRef]
- Armannsson, H.; Fridriksson, T.; Kristjánsson, B.R. CO2 emissions from geothermal power plants and natural geothermal activity in Iceland. Geothermics 2005, 34, 286–296. [Google Scholar] [CrossRef]
- Hammons, T.J. Geothermal power generation worldwide: Global perspective, technology, field experience, and research and development. Electric Power Compon. Syst. 2004, 32, 529–553. [Google Scholar] [CrossRef]
- Diaz, A.R.; Kaya, E.; Zarrouk, S.J. Reinjection in geothermal fields—A worldwide review update. Renew. Sust. Energ. Rev. 2016, 53, 105–162. [Google Scholar] [CrossRef]
- Kaya, E.; Zarrouk, S.J. Reinjection of greenhouse gases into geothermal reservoirs. Int. J. Greenh. Gas Control. 2017, 67, 111–129. [Google Scholar] [CrossRef]
- Fiaschi, D.; Manfrida, G.; Niknam, P.; Talluri, L. Sensitivity Analysis of the Total Reinjection Geothermal Plant in Castelnuovo. In Proceedings of the 7th European Geothermal Workshop—Characterization of Deep Geothermal Systems, Karlsruhe, Germany, 9–10 October 2019. [Google Scholar]
- Thorhallsson, S. Common Problems Faced in Geothermal Generation and How to Deal with Them. In Proceedings of the Workshop for Decision Makers on Geothermal Projects and Management Naivasha, Santa Tecla, El Salvador, 11–17 March 2012. [Google Scholar]
- Savary, V.; Berger, G.; Dubois, M.; Lacharpagne, J.C.; Pages, A.; Thibeau, S.; Lescanne, M. The solubility of CO2+ H2S mixtures in water and 2 M NaCl at 120° C and pressures up to 35 MPa. Int. J. Greenh. Gas Control. 2012, 10, 123–133. [Google Scholar] [CrossRef]
- Shabani, B.; Vilcáez, J. Prediction of CO2-CH4-H2S-N2 gas mixtures solubility in brine using a non-iterative fugacity-activity model relevant to CO2-MEOR. J. Pet Sci. Eng. 2017, 150, 162–179. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Ahmed, R.; Shen, N.; Li, X. An enthalpy model of CO2-CH4-H2S-N2-brine systems applied in simulation of non-isothermal multiphase and multicomponent flow with high pressure, temperature and salinity. J. CO2 Util. 2019, 31, 85–97. [Google Scholar] [CrossRef]
- Afsharpour, A.; Haghtalab, A. Correlation and prediction of H2S and mixture of CO2+ H2S solubility in aqueous MDEA solutions using electrolyte modified HKM plus association EoS. Fluid Phase Equilibr. 2019, 494, 192–200. [Google Scholar] [CrossRef]
- Afsharpour, A.; Haghtalab, A. Implementation of electrolyte CPA EoS to model solubility of CO2 and CO2+ H2S mixtures in aqueous MDEA solutions. Chin. J. Chem. Eng. 2019, 27, 1912–1920. [Google Scholar]
- Cholewinski, A.; Dengis, J.; Malkov, V.; Leonenko, Y. Modeling of CO2 injection into aquifers containing dissolved H2S. J. Nat. Gas Sci. Eng. 2016, 36, 1080–1086. [Google Scholar] [CrossRef]
- Shafaei, M.J.; Abedi, J.; Hassanzadeh, H.; Chen, Z. Reverse gas-lift technology for CO2 storage into deep saline aquifers. Energy 2012, 45, 840–849. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Elsharkawy, A.M.; Keith, D.W.; Leonenko, Y. Predicting PVT data for CO2–brine mixtures for black-oil simulation of CO2 geological storage. Int. J. Greenh. Gas Control. 2008, 2, 65–77. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Keith, D.W. Accelerating CO2 dissolution in saline aquifers for geological storage Mechanistic and sensitivity studies. Energ. Fuel. 2009, 23, 3328–3336. [Google Scholar] [CrossRef]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Phase Equilibr. 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Carroll, J.J.; Slupsky, J.D.; Mather, A.E. The solubility of carbon dioxide in water at low pressure. J. Phys. Chem. Ref. Data 1991, 20, 1201–1209. [Google Scholar] [CrossRef]
- Crovetto, R. Evaluation of solubility data of the system CO2–H2O from 273 K to the critical point of water. J. Phys. Chem. Ref. Data 1991, 20, 575–589. [Google Scholar] [CrossRef] [Green Version]
- Wroblewski, S.V. The solubility of carbon dioxide in water. Ann. Phys. Chem 1883, 18, 290–308. [Google Scholar]
- Anderson, G.K. Solubility of carbon dioxide in water under incipient clathrate formation conditions. J. Phys. Chem. Ref. Data 2002, 47, 219–222. [Google Scholar] [CrossRef]
- Wohlfarth, C.; Wohlfarth, B. Pure Liquids: Extended Data. In Refractive Indices of Organic Liquids; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–747. [Google Scholar]
- Vilcu, R.; Gainar, I. Loslichkeit der gase unter druck in flussigkeiten. i. das system kohlendioxid-wasser. Rev. Roumaine Chim. 1967, 12, 181. [Google Scholar]
- Kritschewsky, I.R.; Shaworonkoff, N.M.; Aepelbaum, V.A. Combined solubility of gases in liquids under pressure: I. Solubility of carbon dioxide in water from its mixtures with hydrogen of 20 and 30 C and total pressure of 30 kg/cm2. Z. Phys. Chem. 1935, 175, 232–238. [Google Scholar]
- Gillespie, P.C.; Wilson, G.M. Vapor-Liquid and Liquid-Liquid Equilibria. Water-Methane, Water-Carbon Dioxide, Water-Hydrogen Sulphide, Water-Pentane. In GPA Research Report; Gas Processors Association: Tulsa, OK, USA, 1982. [Google Scholar]
- Hahnel, O. Solubility of carbon dioxide in water. Centr. Min. Geol. 1920, 25, 25–32. [Google Scholar]
- Stewart, P.B.; Munjal, P.K. Solubility of carbon dioxide in pure water, synthetic sea water, and synthetic sea water concentrates at−5. deg. to 25. deg. and 10-to 45-atm. pressure. J. Chem. Eng. Data 1970, 15, 67–71. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Effect of temperature and pressure on the solubility of carbon dioxide in water in the presence of gas hydrate. Fluid Phase Equilibr. 2001, 190, 127–134. [Google Scholar] [CrossRef]
- Cramer, S.D. Solubility of Methane, Carbon Dioxide, and Oxygen in Brines from 0/sup 0/to 300/sup 0/C; Bureau of Mines: Pittsburgh, PA, USA, 1982. [Google Scholar]
- Teng, H.; Yamasaki, A.; Chun, M.K.; Lee, H. Solubility of liquid CO2 in water at temperatures from 278 K to 293 K and pressures from 6.44 MPa to 29.49 MPa and densities of the corresponding aqueous solutions. J. Chem. Thermodyn. 1997, 29, 1301–1310. [Google Scholar] [CrossRef]
- Shagiakhmetov, R.A.; Tarzimanov, A.A. Measurements of CO2 solubility in water up to 60 MPa. Deposited document SPSTL 200 khp-D 81–1982. 1981. [Google Scholar]
- Scharlin, P.; Cargill, R.W. Carbon Dioxide in Water and Aqueous Electrolyte Solutions. Solubility Data Series; Oxford University Press: Oxford, UK, 1996; Volume 62, p. 383. [Google Scholar]
- Namiot, A.Y.; Bondareva, M.M. Solubility of Gases in Water; Nedra: Moscow, Russia, 1991. [Google Scholar]
- Yang, S.O.; Yang, I.M.; Kim, Y.S.; Lee, C.S. Measurement and prediction of phase equilibria for water+ CO2 in hydrate forming conditions. Fluid Phase Equilibr. 2000, 175, 75–89. [Google Scholar] [CrossRef]
- Zel’vinskii, Y.D. Measurements of carbon dioxide solubility in water. Zhurn. Khim. Prom. 1937, 14, 1250–1257. [Google Scholar]
- Wiebe, R.; Gaddy, V.L. The solubility of carbon dioxide in water at various temperatures from 12 to 40 and at pressures to 500 atmospheres. Critical phenomena. J. Am. Chem. Soc. 1940, 62, 815–817. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L. The solubility in water of carbon dioxide at 50, 75 and 100, at pressures to 700 atmospheres. J. Am. Chem. Soc. 1939, 61, 315–318. [Google Scholar] [CrossRef]
- Bartholomé, E.; Friz, H. Solubility of CO2 in water. Chem. Ing. Technol. 1956, 28, 706–708. [Google Scholar] [CrossRef]
- Zawisza, A.; Malesinska, B. Solubility of carbon dioxide in liquid water and of water in gaseous carbon dioxide in the range 0.2–5 MPa and at temperatures up to 473 K. J. Chem. Eng. Data 1981, 26, 388–391. [Google Scholar] [CrossRef]
- Müller, G.; Bender, E.; Maurer, G. Das Dampf-Flüssigkeitsgleichgewicht des ternären Systems Ammoniak-Kohlendioxid-Wasser bei hohen Wassergehalten im Bereich zwischen 373 und 473 Kelvin. Ber. Bunsenges. Phys. Chem. 1988, 92, 148–160. [Google Scholar] [CrossRef]
- King, M.B.; Mubarak, A.; Kim, J.D.; Bott, T.R. The mutual solubilities of water with supercritical and liquid carbon dioxides. J. Supercrit. Fluids. 1992, 5, 296–302. [Google Scholar] [CrossRef]
- Bamberger, A.; Sieder, G.; Maurer, G. High-pressure (vapor+ liquid) equilibrium in binary mixtures of (carbon dioxide+ water or acetic acid) at temperatures from 313 to 353 K. J. Supercrit. Fluids. 2000, 17, 97–110. [Google Scholar] [CrossRef]
- Lee, J.I.; Mather, A.E. Solubility of hydrogen sulfide in water. Ber. Bunsenges. Phys. Chem. 1977, 81, 1020–1023. [Google Scholar] [CrossRef]
- Honeywell International Inc. UniSim® Design, Operations guide, R460 release, Honeywell: Rolle, Switzerland. 2017. Available online: www.hwll.co/uniSimDesign (accessed on 1 December 2019).
- Niknam, P.; Fiaschi, D.; Mortaheb, H.R.; Mokhtarani, B. An improved formulation for speed of sound in two-phase systems and development of 1D model for supersonic nozzle. Fluid Phase Equilibr. 2017, 446, 18–27. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Valtz, A.; Chapoy, A.; Coquelet, C.; Paricaud, P.; Richon, D. Vapour–liquid equilibria in the carbon dioxide-water system, measurement and modelling from 278.2 to 318.2K. Fluid Phase Equilib. 2004, 226, 333–344. [Google Scholar] [CrossRef]
- Gu, F.Y. Solubility of carbon dioxide in aqueous sodium chloride solution under high pressure. J. Chem. Eng. Chin. Univ. 1998, 12, 118–123. (In Chinese) [Google Scholar]
- Tang, Y.; Bian, X.; Du, Z.; Wang, C. Measurement and prediction model of carbon dioxide solubility in aqueous solutions containing bicarbonate anion. Fluid Phase Equilibr. 2015, 386, 56–64. [Google Scholar] [CrossRef]
- Chapoy, A.; Mohammadi, A.H.; Chareton, A.; Tohidi, B.; Richon, D. Measurement and modeling of gas solubility and literature review of the properties for the carbon dioxide—Water system. Ind. Eng. Chem. Res. 2004, 43, 1794–1802. [Google Scholar] [CrossRef]
- Hou, S.-X.; Maitland, G.C.; Trusler, J.P.M. Measurement and modeling of the phase behavior of the (carbon dioxide plus water) mixture at temperatures from 298.15 K to 448.15 K. J. Supercrit. Fluids. 2013, 73, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Pfohl, O.; Dohrn, R.; Brunner, G. Partitioning of Carbohydrates in the Three-Phase Region of Systems Containing Carbon Dioxide, Water and a Modifier at High Pressure. In Process Technology Proceedings; Elsevier: Amsterdam, The Netherlands, 1996; Volume 12, pp. 277–282. [Google Scholar]










| Method | Approach | Advantages | Disadvantages | Application |
|---|---|---|---|---|
| Thermodynamic models | Two-phase equilibrium-state calculation | -High accuracy -Valid for a wide range of pressures and temperatures | -High computational cost -Dependency on the EoS -Partial inconsistency with the reinjection | All |
| PVT dataset [15,16,17] | Lookup-table properties | -Simple calculation by interpolation -Commercially used and already evaluated -Possibility of extrapolation | -Limited range of P, T or the composition -Partial inconsistency with the reinjection | Mainly available for oil and gas case studies |
| Literature correlations [18] | Deriving a formulation by using reference data | -Quick estimation -Ability to calculate the solubility in or out of the reference data -The accuracy of the model can be improved by adapting the form of the equation. | -Partial inconsistency with the reinjection -Limited to the solubility of pure gas in water or brine (e.g., CO2 in water or H2S in water) | Depends on the reference experimental data |
| Proposed correlation | -All of the advantages of the literature correlations -High reliability due to a large amount of reference data including both experimental and the thermodynamic-model data -Covering a wide range of P and T -Adapted to the physics of the injection process -Applicable for pure gas solubility -Applicable for mixture gas solubility and taking into account the interactions. | Limited to the solubility of CO2-H2S mixture in water | Reinjection of the CO2-H2S mixture in geothermal power plants. |
| Type of Data | Reference | Pressure (MPa) | Temperature (°C) | Data N° |
|---|---|---|---|---|
| CO2 in water | Diamond, 2003 [18] | 0.1–100 | 0–100 | 520 |
| H2S in water | Lee, 1977 [44] | 0–6.67 | 10–180 | 100 |
| Binary gas (CO2 + H2S) in water | Savary, 2012 [9] | 3.9–35 | 120 (fixed) | 50 |
| Binary gas (CO2 + H2S) in water | UniSim® [45] | 0–15 | 0–150 | 190000 |
| Index | Coefficients for CO2 (ai) | Coefficients for H2S (bi) |
|---|---|---|
| 1 | 5.1392 × 10−1 | 2.4812 × 10−1 |
| 2 | 4.7999 × 10+1 | 8.0370 |
| 3 | 3.0091 × 10+1 | 7.3159 × 10+1 |
| 4 | 6.3701 × 10+2 | 8.7230 × 10+1 |
| 5 | −6.7389 × 10−1 | −6.1670 × 10−1 |
| 6 | 1.0548 × 10−1 | 1.9911 × 10−1 |
| 7 | 2.6740 | 2.0354 |
| 8 | −7.9216 × 10−1 | −1.0341 |
| 9 | 5.9794 | 4.1092 |
| 10 | 7.0812 × 10−1 | 7.4443 × 10−1 |
| 11 | 2.1386 × 10+1 | 2.4380 × 10+1 |
| 12 | 7.1616 × 10−1 | 1.2383 × 10+2 |
| 13 | 5.4354 × 10+1 | 1.7089 × 10+1 |
| 14 | 7.0093 × 10−1 | 7.4445 × 10−1 |
| Case | R2 | MSE | MAE |
|---|---|---|---|
| CO2 in water | 0.9803 | 6.11 × 10−6 | 1.067 × 10−3 |
| H2S in water | 0.9833 | 2.10 × 10−6 | 5.331 × 10−4 |
| Partial solubility of CO2 in water | 0.9529 | 2.90 × 10−5 | 1.326 × 10−3 |
| Partial solubility of H2S in water | 0.9733 | 7.79 × 10−4 | 5.226 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
H. Niknam, P.; Talluri, L.; Fiaschi, D.; Manfrida, G. Improved Solubility Model for Pure Gas and Binary Mixture of CO2-H2S in Water: A Geothermal Case Study with Total Reinjection. Energies 2020, 13, 2883. https://doi.org/10.3390/en13112883
H. Niknam P, Talluri L, Fiaschi D, Manfrida G. Improved Solubility Model for Pure Gas and Binary Mixture of CO2-H2S in Water: A Geothermal Case Study with Total Reinjection. Energies. 2020; 13(11):2883. https://doi.org/10.3390/en13112883
Chicago/Turabian StyleH. Niknam, Pouriya, Lorenzo Talluri, Daniele Fiaschi, and Giampaolo Manfrida. 2020. "Improved Solubility Model for Pure Gas and Binary Mixture of CO2-H2S in Water: A Geothermal Case Study with Total Reinjection" Energies 13, no. 11: 2883. https://doi.org/10.3390/en13112883