Facile Electrodeposition of Poly(3,4-ethylenedioxythiophene) on Poly(vinyl alcohol) Nanofibers as the Positive Electrode for High-Performance Asymmetric Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA Nanofiber via Electrospinning
2.3. Electrodeposition of PEDOT on PVA Nanofibers
2.4. Reduction of GO
2.5. Characterizations
3. Results and Discussion
3.1. Structural Morphology
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
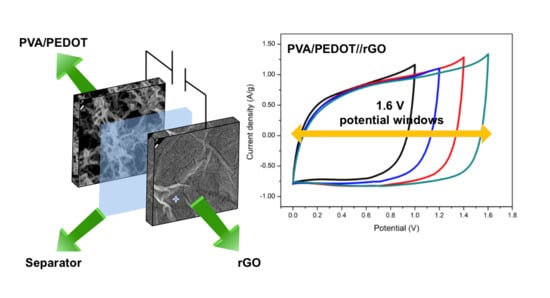
3.3. Electrochemical Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajkumar, M.; Hsu, C.-T.; Wu, T.-H.; Chen, M.-G.; Hu, C.-C. Advanced materials for aqueous supercapacitors in the asymmetric design. Prog. Nat. Sci.-Mater. Int. 2015, 25, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Subramania, A.; Balakrishnan, K. Preparation of electrospun Co3O4 nanofibers as electrode material for high performance asymmetric supercapacitors. Electrochim. Acta 2014, 149, 152–158. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Zubair, N.A.; Azman, N.H.N.; Sulaiman, Y. Fabrication of PEDOT coated PVA-GO nanofiber for supercapacitor. Mater. Chem. Phys. 2017, 192, 161–169. [Google Scholar] [CrossRef]
- Kiran, S.K.; Padmini, M.; Das, H.T.; Elumalai, P. Performance of asymmetric supercapacitor using CoCr-layered double hydroxide and reduced graphene-oxide. J. Solid State Electrochem. 2017, 21, 927–938. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Kumar, Y.; Chandra, R. An efficient α-MnO2 nanorods forests electrode for electrochemical capacitors with neutral aqueous electrolytes. Electrochim. Acta 2016, 220, 712–720. [Google Scholar] [CrossRef]
- Mohammadi, A.; Arsalani, N.; Tabrizi, A.G.; Moosavifard, S.E.; Naqshbandi, Z.; Ghadimi, L.S. Engineering rGO-CNT wrapped Co3S4 nanocomposites for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 66–80. [Google Scholar] [CrossRef]
- Ning, P.; Duan, X.; Ju, X.; Lin, X.; Tong, X.; Pan, X.; Wang, T.; Li, Q. Facile synthesis of carbon nanofibers/MnO2 nanosheets as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 2016, 210, 754–761. [Google Scholar] [CrossRef]
- Bhagwan, J.; Sivasankaran, V.; Yadav, K.L.; Sharma, Y. Porous, one-dimensional and high aspect ratio nanofibric network of cobalt manganese oxide as a high performance material for aqueous and solid-state supercapacitor (2 V). J. Power Sources 2016, 327, 29–37. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Luo, M.; Lei, Z. High-performance aqueous asymmetric supercapacitor based on carbon nanofibers network and tungsten trioxide nanorod bundles electrodes. Electrochim. Acta 2014, 147, 54–61. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, J.; Chen, L.; Tang, S.; Deng, M.; Du, Y. All-solid-state asymmetric supercapacitors based on Fe-doped mesoporous Co3O4 and three-dimensional reduced graphene oxide electrodes with high energy and power densities. Nanoscale 2017, 9, 15423–15433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cai, D.; Zhan, H. Construction of reduced graphene oxide nanofibers and cobalt sulfide nanocomposite for pseudocapacitors with enhanced performance. J. Alloys Compd. 2017, 706, 126–132. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Fernández-Garcia, L.; González, Z.; Granda, M.; Menendez, R.; Gryglewicz, G. MnO2/thermally reduced graphene oxide composites for high-voltage asymmetric supercapacitors. Electrochim. Acta 2017, 240, 53–62. [Google Scholar] [CrossRef]
- Jiang, L.; Sui, Y.; Qi, J.; Chang, Y.; He, Y.; Meng, Q.; Wei, F.; Sun, Z.; Jin, Y. Hierarchical Ni-Co layered double hydroxide nanosheets on functionalized 3D-RGO films for high energy density asymmetric supercapacitor. Appl. Surf. Sci. 2017, 426, 148–159. [Google Scholar] [CrossRef]
- Lamiel, C.; Lee, Y.R.; Cho, M.H.; Tuma, D.; Shim, J.-J. Enhanced electrochemical performance of nickel-cobalt-oxide@reduced graphene oxide//activated carbon asymmetric supercapacitors by the addition of a redox-active electrolyte. J. Colloid Interface Sci. 2017, 507, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, B.; Hou, H.; Wu, L.; Zhu, X.; Hu, J.; Yang, J. Facile preparation of flower-like NiMn layered double hydroxide/reduced graphene oxide microsphere composite for high-performance asymmetric supercapacitors. J. Alloys Compd. 2018, 730, 71–80. [Google Scholar] [CrossRef]
- Mao, X.; Yang, W.; He, X.; Chen, Y.; Zhao, Y.; Zhou, Y.; Yang, Y.; Xu, J. The preparation and characteristic of poly (3,4-ethylenedioxythiophene)/reduced graphene oxide nanocomposite and its application for supercapacitor electrode. Mater. Sci. Eng. B 2017, 216, 16–22. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Li, M.; Zhang, L.; Huang, Y.; Hu, X. Assembly of NiO/Ni(OH)2/PEDOT Nanocomposites on Contra Wires for Fiber-Shaped Flexible Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 1774–1779. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Shin, D.Y.; An, G.H.; Ahn, H.J. Barnacle-like manganese oxide decorated porous carbon nanofibers for high-performance asymmetric supercapacitors. Ceram. Int. 2018, 44, 4883–4890. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Fu, W.; Zhang, P.; Pan, X.; Xie, E. In situ synthesis of CoSx@carbon core-shell nanospheres decorated in carbon nanofibers for capacitor electrodes with superior rate and cycling performances. Carbon 2017, 114, 187–197. [Google Scholar] [CrossRef]
- Zhao, P.; Yao, M.; Ren, H.; Wang, N.; Komarneni, S. Nanocomposites of hierarchical ultrathin MnO2 nanosheets/hollow carbon nanofibers for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2019, 463, 931–938. [Google Scholar] [CrossRef]
- Zubair, N.A.; Rahman, N.A.; Lim, H.N.; Sulaiman, Y. Production of Conductive PEDOT-Coated PVA-GO Composite Nanofibers. Nanoscale Res. Lett. 2017, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly(vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Pan, Q.; Tong, N.; He, N.; Liu, Y.; Shim, E.; Pourdeyhimi, B.; Gao, W. Electrospun Mat of Poly(vinyl alcohol)/Graphene Oxide for Superior Electrolyte Performance. ACS Appl. Mater. Interfaces 2018, 10, 7927–7934. [Google Scholar] [CrossRef] [PubMed]
- Syed Zainol Abidin, S.N.J.; Mamat, S.; Abdul Rasyid, S.; Zainal, Z.; Sulaiman, Y. Fabrication of poly(vinyl alcohol)-graphene quantum dots coated with poly(3,4-ethylenedioxythiophene) for supercapacitor. J. Polym. Sci. A 2017, 56, 50–58. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.-H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Yohannes, T.; Carlberg, J.C.; Inganäs, O.; Solomon, T. Electrochemical and spectroscopic characteristics of copolymers electrochemically synthesized from 3-methylthiophene and 3,4-ethylenedioxy thiophene. Synth. Met. 1997, 88, 15–21. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, F.; Zeng, B. Electrodeposition of poly(3,4-ethylenedioxythiophene) on a stainless steel wire for solid phase microextraction and GC determination of some esters with high boiling points. Talanta 2013, 104, 27–31. [Google Scholar] [CrossRef]
- Bonanni, A.; Ambrosi, A.; Pumera, M. On oxygen-containing groups in chemically modified graphenes. Chem. Eur. J. 2012, 18, 4541–4548. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.Ö.; Ekinci, D.; Demir, Ü. Atomic scale imaging and spectroscopic characterization of electrochemically reduced graphene oxide. Surf. Sci 2013, 611, 54–59. [Google Scholar] [CrossRef]
- Lin, S.-C.; Lu, Y.-T.; Chien, Y.-A.; Wang, J.-A.; You, T.-H.; Wang, Y.-S.; Lin, C.-W.; Ma, C.-C.M.; Hu, C.-C. Asymmetric supercapacitors based on functional electrospun carbon nanofiber/manganese oxide electrodes with high power density and energy density. J. Power Sources 2017, 362, 258–269. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Wang, H.; Shao, H.; Fang, J.; He, J.; Xiong, H.; Ma, C.; Lin, T. High-performance supercapacitor electrode from cellulose-derived, inter-bonded carbon nanofibers. J. Power Sources 2016, 324, 302–308. [Google Scholar] [CrossRef]
- Shivakumara, S.; Munichandraiah, N. Asymmetric supercapacitor based on nanostructured porous manganese oxide and reduced graphene oxide in aqueous neutral electrolyte. Solid State Commun. 2017, 260, 34–39. [Google Scholar] [CrossRef]
- 3Cho, S.; Patil, B.; Yu, S.; Ahn, S.; Hwang, J.; Park, C.; Do, K.; Ahn, H. Flexible, Swiss roll, fiber-shaped, asymmetric supercapacitor using MnO2 and Fe2O3 on carbon fibers. Electrochim. Acta 2018, 269, 499–508. [Google Scholar]
- Ye, A.; Sui, Y.; Qi, J.; Wei, F.; He, Y.; Meng, Q.; Ren, Y.; Sun, Z. Self-Supported Ni0.85Se Nanosheets Array on Carbon Fiber Cloth for a High-Performance Asymmetric Supercapacitor. J. Electron. Mater. 2018, 47, 7002–7010. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, H.; Xu, X.; Shi, J.; Liu, W.; Wang, X. Balanced mesoporous nickle cobaltite-graphene and doped carbon electrodes for high-performance asymmetric supercapacitor. Chem. Eng. J. 2017, 326, 401–410. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.-F. Graphene-beaded carbon nanofibers for use in supercapacitor electrodes: Synthesis and electrochemical characterization. J. Power Sources 2013, 222, 410–416. [Google Scholar] [CrossRef]
- Rajesh, M.; Raj, J.; Manikandan, R.; Kim, B.C.; Yeup Park, S.; Hyun Yu, K. A High Performance PEDOT/PEDOT Symmetric Supercapacitor by Facile in-situ Hydrothermal Polymerization of PEDOT Nanostructures on Flexible Carbon Fibre Cloth Electrodes. Materi. Today Energy 2017, 6, 96–104. [Google Scholar] [CrossRef]
- Ravit, R.; Abdullah, J.; Ahmad, I.; Sulaiman, Y. Electrochemical performance of poly(3, 4-ethylenedioxythipohene)/nanocrystalline cellulose (PEDOT/NCC) film for supercapacitor. Carbohydr. Polym. 2019, 203, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Li, Y.; Liu, D.; Gu, Y.; Qin, S.; Guo, X.; Guo, H.; Ding, Y.; Liu, Q.; Chen, Q.; et al. High-performance free-standing capacitor electrodes of multilayered Co9S8 plates wrapped by carbonized poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate)/reduced graphene oxide. J. Power Sources 2018, 379, 167–173. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, H.; Zhang, M.; Li, L.; Wang, G.; Shan, X. Three-dimensional porous ZnCo2O4 sheet array coated with Ni(OH)2 for high-performance asymmetric supercapacitor. J. Colloid Interface Sci. 2017, 497, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, G.; Zhang, S.; Zhang, Y.X. Decoration of carbon cloth by manganese oxides for flexible asymmetric supercapacitors. Ceram. Int. 2017, 43, 8321–8328. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, Y. Pulse Electrodeposition of Manganese Oxide for High-Rate Capability Supercapacitors. Int. J. Electrochem. Sci. 2012, 7, 7440–7450. [Google Scholar]
- Huang, Y.; Shi, T.; Jiang, S.; Cheng, S.; Tao, X.; Zhong, Y.; Liao, G.; Tang, Z. Enhanced cycling stability of NiCo2S4@NiO core-shell nanowire arrays for all-solid-state asymmetric supercapacitors. Sci. Rep. 2016, 6, 38620. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat. Commun. 2015, 6, 8503. [Google Scholar] [CrossRef]
- Li, Q.; Lu, C.; Chen, C.; Xie, L.; Liu, Y.; Li, Y.; Kong, Q.; Wang, H. Layered NiCo2O4/reduced graphene oxide composite as an advanced electrode for supercapacitor. Energy Storage Mater. 2017, 8, 59–67. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasdevan, N.; Mohd Abdah, M.A.A.; Sulaiman, Y. Facile Electrodeposition of Poly(3,4-ethylenedioxythiophene) on Poly(vinyl alcohol) Nanofibers as the Positive Electrode for High-Performance Asymmetric Supercapacitor. Energies 2019, 12, 3382. https://doi.org/10.3390/en12173382
Dasdevan N, Mohd Abdah MAA, Sulaiman Y. Facile Electrodeposition of Poly(3,4-ethylenedioxythiophene) on Poly(vinyl alcohol) Nanofibers as the Positive Electrode for High-Performance Asymmetric Supercapacitor. Energies. 2019; 12(17):3382. https://doi.org/10.3390/en12173382
Chicago/Turabian StyleDasdevan, Nivekthiren, Muhammad Amirul Aizat Mohd Abdah, and Yusran Sulaiman. 2019. "Facile Electrodeposition of Poly(3,4-ethylenedioxythiophene) on Poly(vinyl alcohol) Nanofibers as the Positive Electrode for High-Performance Asymmetric Supercapacitor" Energies 12, no. 17: 3382. https://doi.org/10.3390/en12173382