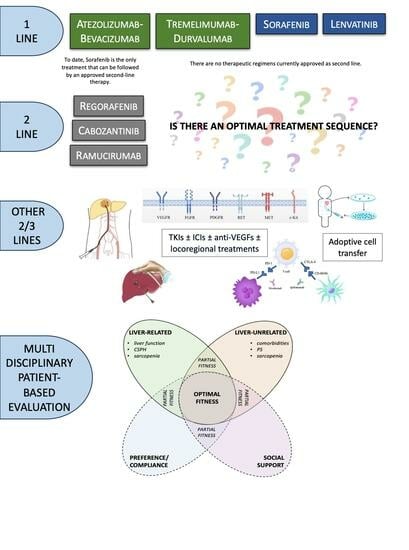
The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence
Abstract
:1. Introduction
2. First-Line Approach to HCC
2.1. Currently Approved First-Line Therapies
2.2. How to Choose the First-Line Systemic Treatment
3. Second-Line Approach to HCC
3.1. Currently Approved Second-Line Therapies Post Sorafenib
3.2. Second-Line Options Post Lenvatinib
3.3. Second-Line Options Post Atezolizumab-Bevacizumab
4. Current and Future Perspectives on Systemic Treatment for HCC
4.1. Is There an Optimal Treatment Sequence?
4.2. How to Choose the Most Feasible Treatment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Su, G.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shen, Y.; Cheng, A. Evolution of Systemic Treatment for Advanced Hepatocellular Carcinoma. Kaohsiung J. Med. Sci. 2021, 37, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Uson Junior, P.; Liu, A.; Sonbol, M.; Borad, M.; Bekaii-Saab, T. Immunotherapy and Chimeric Antigen Receptor T-Cell Therapy in Hepatocellular Carcinoma. Chin. Clin. Oncol. 2021, 10, 11. [Google Scholar] [CrossRef]
- Vitale, A.; Cabibbo, G.; Iavarone, M.; Viganò, L.; Pinato, D.J.; Ponziani, F.R.; Lai, Q.; Casadei-Gardini, A.; Celsa, C.; Galati, G.; et al. Personalised Management of Patients with Hepatocellular Carcinoma: A Multiparametric Therapeutic Hierarchy Concept. Lancet Oncol. 2023, 24, e312–e322. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.; Qin, S.; Han, K.; Ikeda, K. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, C.; Talbot, T.; Murray, S.; Silletta, M.; Vincenzi, B.; Cortellini, A.; Pinato, D. Immunotherapy in Hepatocellular Carcinoma. Curr. Treat. Options Oncol. 2021, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 Randomized, Open-Label, Multicenter Study of Tremelimumab (T) and Durvalumab (D) as First-Line Therapy in Patients (Pts) with Unresectable Hepatocellular Carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.; Kelley, R.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Toni, E.D.; Furuse, J.; Kang, Y.; Galle, P.; et al. SO-15 Four-Year Overall Survival Update from the Phase 3 HIMALAYA Study of Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. Ann. Oncol. 2023, 34, S168. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tremelimumab-combination-durvalumab-unresectable-hepatocellular-carcinoma (accessed on 12 September 2023).
- Maestri, M.; Pallozzi, M.; Santopaolo, F.; Cerrito, L.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Durvalumab: An Investigational Agent for Unresectable Hepatocellular Carcinoma. Expert Opin. Investig. Drugs 2022, 31, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Riaz, I.B.; Naqvi, S.A.A.; Almquist, D.R.; Mina, S.; Almasri, J.; Shah, S.; Almader-Douglas, D.; Uson Junior, P.L.S.; Mahipal, A.; et al. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-Analysis. JAMA Oncol. 2020, 6, e204930. [Google Scholar] [CrossRef]
- Persano, M.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Clinical Outcomes with Atezolizumab plus Bevacizumab or Lenvatinib in Patients with Hepatocellular Carcinoma: A Multicenter Real-World Study. J. Cancer Res. Clin. Oncol. 2023, 149, 5591–5602. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus Bevacizumab versus Lenvatinib for Unresectable Hepatocellular Carcinoma: A Large Real-Life Worldwide Population. Eur. J. Cancer Oxf. Engl. 2023, 180, 9–20. [Google Scholar] [CrossRef]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus Bevacizumab versus Lenvatinib or Sorafenib in Non-Viral Unresectable Hepatocellular Carcinoma: An International Propensity Score Matching Analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef]
- Rimini, M.; Persano, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Presa, J.; et al. Survival Outcomes from Atezolizumab plus Bevacizumab versus Lenvatinib in Child Pugh B Unresectable Hepatocellular Carcinoma Patients. J. Cancer Res. Clin. Oncol. 2023, 149, 7565–7577. [Google Scholar] [CrossRef]
- Fulgenzi, C.A.M.; D’Alessio, A.; Airoldi, C.; Scotti, L.; Demirtas, C.O.; Gennari, A.; Cortellini, A.; Pinato, D.J. Comparative Efficacy of Novel Combination Strategies for Unresectable Hepatocellular Carcinoma: A Network Metanalysis of Phase III Trials. Eur. J. Cancer 2022, 174, 57–67. [Google Scholar] [CrossRef]
- Hatanaka, T.; Naganuma, A.; Yata, Y.; Kakizaki, S. Atezolizumab plus Bevacizumab and Tremelimumab plus Durvalumab: How Should We Choose These Two Immunotherapy Regimens for Advanced Hepatocellular Carcinoma? Hepatobiliary Surg. Nutr. 2022, 11, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic Treatment of Hepatocellular Carcinoma: An EASL Position Paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Piñero, F.; Silva, M.; Iavarone, M. Sequencing of Systemic Treatment for Hepatocellular Carcinoma: Second Line Competitors. World J. Gastroenterol. 2020, 26, 1888–1900. [Google Scholar] [CrossRef]
- Amaro, C.; Tam, V. Management of Hepatocellular Carcinoma after Progression on First-Line Systemic Treatment: Defining the Optimal Sequencing Strategy in Second Line and Beyond. Curr. Oncol. 2020, 27, 173–180. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.; Bodoky, G. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (Resorce): A Randomised, Double-Blind, Placebo- Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Gerolami, R.; Caparello, C.; et al. Outcomes of Sequential Treatment with Sorafenib Followed by Regorafenib for HCC: Additional Analyses from the Phase III RESORCE Trial. J. Hepatol. 2018, 69, 353–358. [Google Scholar] [CrossRef]
- Abou-Alfa, G.; Meyer, T.; Cheng, A.; El-Khoueiry, A.; Rimassa, L.; Ryoo, B.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Hanna, D.L.; Llovet, J.; Kelley, R.K. Cabozantinib: An Evolving Therapy for Hepatocellular Carcinoma. Cancer Treat. Rev. 2021, 98, 102221. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.; Yen, C.; Finn, R.; Galle, P.R. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z.; et al. Apatinib as Second-Line or Later Therapy in Patients with Advanced Hepatocellular Carcinoma (AHELP): A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Zhu, K.; Ren, Z.-G.; Zhou, J.; Fan, J.; Gao, Q. A Review of 2022 Chinese Clinical Guidelines on the Management of Hepatocellular Carcinoma: Updates and Insights. Hepatobiliary Surg. Nutr. 2023, 12, 216–228. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.H.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Updated Efficacy and Safety of KEYNOTE-224: A Phase II Study of Pembrolizumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib. Eur. J. Cancer 2022, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Persano, M.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Presa, J.; et al. Sequential Therapies after Atezolizumab plus Bevacizumab or Lenvatinib First-Line Treatments in Hepatocellular Carcinoma Patients. Eur. J. Cancer 2023, 189, 112933. [Google Scholar] [CrossRef] [PubMed]
- Cabibbo, G.; Celsa, C.; Enea, M.; Battaglia, S.; Rizzo, G.E.M.; Grimaudo, S.; Matranga, D.; Attanasio, M.; Bruzzi, P.; Craxì, A.; et al. Optimizing Sequential Systemic Therapies for Advanced Hepatocellular Carcinoma: A Decision Analysis. Cancers 2020, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Alsina, A.; Kudo, M.; Vogel, A.; Cheng, A.-L.; Tak, W.Y.; Ryoo, B.-Y.; Evans, T.R.J.; López López, C.; Daniele, B.; Misir, S.; et al. Effects of Subsequent Systemic Anticancer Medication Following First-Line Lenvatinib: A Post Hoc Responder Analysis from the Phase 3 REFLECT Study in Unresectable Hepatocellular Carcinoma. Liver Cancer 2020, 9, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kudo, M.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Tsurusaki, M.; et al. Exploratory Analysis of Lenvatinib Therapy in Patients with Unresectable Hepatocellular Carcinoma Who Have Failed Prior PD−1/PD-L1 Checkpoint Blockade. Cancers 2020, 12, 3048. [Google Scholar] [CrossRef]
- Cabibbo, G.; Reig, M.; Celsa, C.; Torres, F.; Battaglia, S.; Enea, M.; Rizzo, G.E.M.; Petta, S.; Calvaruso, V.; Di Marco, V.; et al. First-Line Immune Checkpoint Inhibitor-Based Sequential Therapies for Advanced Hepatocellular Carcinoma: Rationale for Future Trials. Liver Cancer 2022, 11, 75–84. [Google Scholar] [CrossRef]
- Chen, C.-T.; Feng, Y.-H.; Yen, C.-J.; Chen, S.-C.; Lin, Y.-T.; Lu, L.-C.; Hsu, C.-H.; Cheng, A.-L.; Shao, Y.-Y. Prognosis and Treatment Pattern of Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab and Bevacizumab Treatment. Hepatol. Int. 2022, 16, 1199–1207. [Google Scholar] [CrossRef]
- Cabibbo, G.; Aghemo, A.; Lai, Q.; Masarone, M.; Montagnese, S.; Ponziani, F.R. Optimizing Systemic Therapy for Advanced Hepatocellular Carcinoma: The Key Role of Liver Function. Dig. Liver Dis. 2022, 54, 452–460. [Google Scholar] [CrossRef]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-C.; Zhu, X.-D. Downstaging Conversion Therapy in Patients With Initially Unresectable Advanced Hepatocellular Carcinoma: An Overview. Front. Oncol. 2021, 11, 772195. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Kawamura, Y.; Kobayashi, Y.; Kobayashi, M.; Akuta, N.; Okubo, S.; Suzuki, Y.; Hashimoto, M. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann. Surg. Oncol. 2021, 28, 7663–7672. [Google Scholar] [CrossRef]
- Han, Y.; Zhi, W.-H.; Xu, F.; Zhang, C.-B.; Huang, X.-Q.; Luo, J.-F. Selection of First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis of Randomized Controlled Trials. World J. Gastroenterol. 2021, 27, 2415–2433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-D.; Huang, C.; Shen, Y.-H.; Ji, Y.; Ge, N.-L.; Qu, X.-D.; Chen, L.; Shi, W.-K.; Li, M.-L.; Zhu, J.-J.; et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer 2021, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org; ESMO Guidelines Committee Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Kudo, M. Sequential Therapy for Hepatocellular Carcinoma after Failure of Atezolizumab plus Bevacizumab Combination Therapy. Liver Cancer 2021, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.S.L.; Kwok, G.G.W.; Tang, V.; Li, B.C.W.; Leung, R.; Chiu, J.; Ma, K.W.; She, W.H.; Tsang, J.; Lo, C.M.; et al. Ipilimumab and Nivolumab/Pembrolizumab in Advanced Hepatocellular Carcinoma Refractory to Prior Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e001945. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Golan, T.; Dahan, L.; Fu, S.; Moreno, V.; Park, K.; Geva, R.; Braud, F.D.; Wainberg, Z.A.; Reck, M.; et al. Ramucirumab and Durvalumab for Previously Treated, Advanced Non–Small-Cell Lung Cancer, Gastric/Gastro-Oesophageal Junction Adenocarcinoma, or Hepatocellular Carcinoma: An Open-Label, Phase Ia/b Study (JVDJ). Eur. J. Cancer 2020, 137, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.-Y.; Hsu, C.-H.; Li, D.; Burgoyne, A.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Rao Edubilli, T.; et al. Results from the MORPHEUS-Liver Study: Phase Ib/II Randomized Evaluation of Tiragolumab (Tira) in Combination with Atezolizumab (Atezo) and Bevacizumab (Bev) in Patients with Unresectable, Locally Advanced or Metastatic Hepatocellular Carcinoma (uHCC). J. Clin. Oncol. 2023, 41, 4010. [Google Scholar] [CrossRef]
- Liu, J.K.H.; Irvine, A.F.; Jones, R.L.; Samson, A. Immunotherapies for Hepatocellular Carcinoma. Cancer Med. 2022, 11, 571–591. [Google Scholar] [CrossRef]
- Kudo, M. Adjuvant Atezolizumab-Bevacizumab after Resection or Ablation for Hepatocellular Carcinoma. Liver Cancer 2023, 12, 189–197. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.Gov. 2019. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04191889 (accessed on 10 August 2023).
- US National Library of Medicine. ClinicalTrials.Gov. 2016. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02821754 (accessed on 10 August 2023).
- US National Library of Medicine. ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04220944 (accessed on 10 August 2023).
- US National Library of Medicine. ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05220020 (accessed on 10 August 2023).
- US National Library of Medicine. ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03937830 (accessed on 10 August 2023).
- Khaled, N.B.; Seidensticker, M.; Ricke, J.; Mayerle, J.; Oehrle, B.; Rössler, D.; Teupser, D.; Ehmer, U.; Bitzer, M.; Waldschmidt, D.; et al. Atezolizumab and Bevacizumab with Transarterial Chemoembolization in Hepatocellular Carcinoma: The DEMAND Trial Protocol. Future Oncol. 2022, 18, 1423–1435. [Google Scholar] [CrossRef]
- Park, M.K.; Yu, S.J. Concurrent Transarterial Radioembolization and Combination Atezolizumab/ Bevacizumab Treatment of Infiltrative Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: A Case Report. J. Liver Cancer 2022, 22, 69–74. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04541173 (accessed on 10 August 2023).
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) plus Sorafenib as Compared with TACE Alone in Patients with Hepatocellular Carcinoma: TACTICS Trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, Y.J.; Kim, D.Y.; Bae, S.-H.; Paik, S.W.; Lee, Y.-J.; Kim, H.Y.; Lee, H.C.; Han, S.Y.; Cheong, J.Y.; et al. Sorafenib with or without Concurrent Transarterial Chemoembolization in Patients with Advanced Hepatocellular Carcinoma: The Phase III STAH Trial. J. Hepatol. 2019, 70, 684–691. [Google Scholar] [CrossRef]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; De Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of Combined Selective Internal Radiation Therapy and Sorafenib on Survival in Advanced Hepatocellular Carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z.; et al. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J. Clin. Oncol. 2023, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.-L.; Bronowicki, J.-P.; Chen, X.-P.; Dagher, L.; Furuse, J.; Geschwind, J.-F.H.; De Guevara, L.L.; et al. Observational Registry of Sorafenib Use in Clinical Practice across Child-Pugh Subgroups: The GIDEON Study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Ponziani, F.R.; Pompili, M.; Bria, E.; Tortora, G.; Gasbarrini, A.; et al. Skeletal Muscle Loss during Multikinase Inhibitors Therapy: Molecular Pathways, Clinical Implications, and Nutritional Challenges. Nutrients 2020, 12, 3101. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, S.; Kawaoka, T.; Namba, M.; Kodama, K.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Tsuge, M.; Hiramatsu, A.; et al. Skeletal Muscle Loss during Tyrosine Kinase Inhibitor Treatment for Advanced Hepatocellular Carcinoma Patients. Liver Cancer 2020, 9, 148–155. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Takaguchi, K.; Atsukawa, M.; Itobayashi, E.; Tsuji, K.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Clinical Features of Lenvatinib for Unresectable Hepatocellular Carcinoma in Real-World Conditions: Multicenter Analysis. Cancer Med. 2019, 8, 137–146. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kakizaki, S.; Nagashima, T.; Namikawa, M.; Ueno, T.; Tojima, H.; Takizawa, D.; Naganuma, A.; Arai, H.; Sato, K.; et al. Liver Function Changes in Patients with Hepatocellular Carcinoma Treated with Lenvatinib: Predictive Factors of Progression to Child-Pugh Class B, the Formation of Ascites and the Candidates for the Post-Progression Treatment. Cancers 2020, 12, 2906. [Google Scholar] [CrossRef]
- Iavarone, M.; Cabibbo, G.; Biolato, M.; Della Corte, C.; Maida, M.; Barbara, M.; Basso, M.; Vavassori, S.; Craxì, A.; Grieco, A.; et al. Predictors of Survival in Patients with Advanced Hepatocellular Carcinoma Who Permanently Discontinued Sorafenib. Hepatology 2015, 62, 784. [Google Scholar] [CrossRef]
- Reig, M.; Cabibbo, G. Antiviral Therapy in the Palliative Setting of HCC (BCLC-B and -C). J. Hepatol. 2021, 74, 1225–1233. [Google Scholar] [CrossRef]
- Rimassa, L.; Personeni, N.; Czauderna, C.; Foerster, F.; Galle, P. Systemic Treatment of HCC in Special Populations. J. Hepatol. 2021, 74, 931–943. [Google Scholar] [CrossRef]
- McNamara, M.G.; Slagter, A.E.; Nuttall, C.; Frizziero, M.; Pihlak, R.; Lamarca, A.; Tariq, N.; Valle, J.W.; Hubner, R.A.; Knox, J.J.; et al. Sorafenib as First-Line Therapy in Patients with Advanced Child-Pugh B Hepatocellular Carcinoma—A Meta-Analysis. Eur. J. Cancer 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Patwala, K.; Prince, D.S.; Celermajer, Y.; Alam, W.; Paul, E.; Strasser, S.I.; McCaughan, G.W.; Gow, P.; Sood, S.; Murphy, E.; et al. Lenvatinib for the Treatment of Hepatocellular Carcinoma—A Real-World Multicenter Australian Cohort Study. Hepatol. Int. 2022, 16, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.-P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.; et al. CheckMate 040 Cohort 5: A Phase I/II Study of Nivolumab in Patients with Advanced Hepatocellular Carcinoma and Child-Pugh B Cirrhosis. J. Hepatol. 2021, 75, 600–609. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schönlein, M.; Von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B.; et al. Preliminary Evidence of Safety and Tolerability of Atezolizumab plus Bevacizumab in Patients with Hepatocellular Carcinoma and Child-Pugh A and B Cirrhosis: A Real-world Study. Hepatology 2022, 76, 1000–1012. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.; Furuse, J.; Tak, W.; Masi, G.; Varela, M.; Kim, J.; Tanasanvimon, S.; Reig, M.; Dayyani, F.; et al. O-5 Outcomes by Baseline Liver Function in Patients with Unresectable Hepatocellular Carcinoma Treated with Tremelimumab and Durvalumab in the Phase 3 HIMALAYA Study. Ann. Oncol. 2022, 33, S380–S381. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Fulgenzi, C.A.M.; Cheon, J.; D’Alessio, A.; Nishida, N.; Ang, C.; Marron, T.U.; Wu, L.; Saeed, A.; Wietharn, B.; Cammarota, A.; et al. Reproducible Safety and Efficacy of Atezolizumab plus Bevacizumab for HCC in Clinical Practice: Results of the AB-Real Study. Eur. J. Cancer 2022, 175, 204–213. [Google Scholar] [CrossRef]
- Kudo, M. Selection of Systemic Treatment Regimen for Unresectable Hepatocellular Carcinoma: Does Etiology Matter? Liver Cancer 2022, 11, 283–289. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Chan, S.L. Novel Perspectives in Immune Checkpoint Inhibitors and the Management of Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Cancers 2022, 14, 1526. [Google Scholar] [CrossRef] [PubMed]



| Drug | Mechanism of Action | Trial | Number of Enrolled Patients | Important Exclusion Criteria | Results | Approval |
|---|---|---|---|---|---|---|
| Pembrolizumab | anti PD-1 | KEYNOTE-224, phase II, non-randomized [34] | 156 | Variceal bleeding (esophageal or gastric) or encephalopathy within the previous 6 months, clinically apparent ascites, invasion of main portal vein or inferior vena cava | ORR 18.3%, DCR 61.5% | Approved by the FDA after progression on Sorafenib |
| Nivolumab | anti PD-1 | CheckMate 040, phase I-II, non-randomized [35] | 262 | Any prior or current clinically significant ascites, any history of hepatic encephalopathy | ORR 20%, DCR 64% | Approved by the FDA after progression on Sorafenib |
| Nivolumab + Ipilimumab | anti PD-1 + anti CTLA-4 | CheckMate 040, phase I-II, randomized 1:1:1, no control group [36] | 148 | Any prior or current clinically significant ascites, any history of hepatic encephalopathy | ORR 32%, DCR 54% | Approved by the FDA after progression on Sorafenib |
| Drug | Mechanism of Action | Previous First-Line | Trial | Number of Enrolled Patients | Main Exclusion Criteria |
|---|---|---|---|---|---|
| Atezolizumab + Lenvatinib/Sorafenib vs. Lenvatinib/Sorafenib | anti PD-L1 + TKI vs. TKI | Atezolizumab-Bevacizumab | IMbrave 251, phase III, randomized 1:1, controlled, NCT04770896 | 554 (recruiting) | History of HE, PS ≥ 2, Child–Pugh class worse than A |
| Atezolizumab + Lenvatinib/Cabozantinib vs. Lenvatinib/Cabozantinib | anti PD-L1 + TKI vs. TKI | Atezolizumab-Bevacizumab | ACCRU-GI-2008, phase II, randomized 2:1, controlled, NCT05168163 | 122 (recruiting) | Known co-infection with HBV and HCV, untreated or incompletely treated esophageal/gastric varices at high risk for bleeding, PS ≥ 2, Child–Pugh class worse than A |
| Regorafenib + Pembrolizumab | TKI + anti PD-1 | anti PD-1/PD-L1 | Keynote B70, phase II, non-randomized, NCT04696055 | 95 | Untreated esophageal varices at risk of bleeding, active HBV/HCV co-infection |
| Cabozantinib | TKI | anti PD-1/CTLA-4/PD-1 + CTLA-4 | HCC063, phase II, non-randomized, NCT04588051 | 20 | PS ≥ 3, Child–Pugh class worse than A, concomitant anticoagulation, clinically significant bleeding risk, untreated or incompletely treated varices at high risk for bleeding, moderate or severe ascites |
| Regorafenib + Nivolumab | TKI + anti PD-1 | Sorafenib or Atezolizumab-Bevacizumab | GOING, phase I-II, non-randomized, NCT04170556 | 78 | PS ≥ 2, Child–Pugh class worse than A, clinically meaningful variceal bleeding within the previous 3 months, clinically meaningful ascites, history of HE within the previous 12 months or requirement for medications to prevent or control HE, active HBV/HCV co-infection, concomitant anticoagulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerreto, M.; Cardone, F.; Cerrito, L.; Stella, L.; Santopaolo, F.; Pallozzi, M.; Gasbarrini, A.; Ponziani, F.R. The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence. Curr. Oncol. 2023, 30, 8774-8792. https://doi.org/10.3390/curroncol30100633
Cerreto M, Cardone F, Cerrito L, Stella L, Santopaolo F, Pallozzi M, Gasbarrini A, Ponziani FR. The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence. Current Oncology. 2023; 30(10):8774-8792. https://doi.org/10.3390/curroncol30100633
Chicago/Turabian StyleCerreto, Maria, Ferdinando Cardone, Lucia Cerrito, Leonardo Stella, Francesco Santopaolo, Maria Pallozzi, Antonio Gasbarrini, and Francesca Romana Ponziani. 2023. "The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence" Current Oncology 30, no. 10: 8774-8792. https://doi.org/10.3390/curroncol30100633