Characterization of Dissolved Organic Matter from Agricultural and Livestock Effluents: Implications for Water Quality Monitoring
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of the Experimental Setup
2.2. Sample Collection and Analytical Procedure
2.3. Statistical Analysis
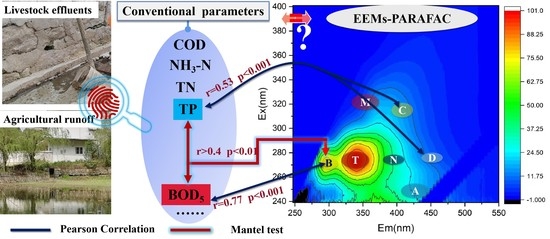
3. Results and Discussion
3.1. Water Chemistry
3.2. Seasonal Variations in DOC, CDOM, and FDOM Concentrations
3.3. EEM-PARAFAC of DOM
3.3.1. Fluorescent PARAFAC Components of DOM
3.3.2. Fluorescence Intensity of DOM
3.3.3. Fluorescence Indices
3.4. Implications for Water Quality Monitoring
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, C.; Wagner, P.D.; Fohrer, N. Effects of land cover, topography, and soil on stream water quality at multiple spatial and seasonal scales in a German lowland catchment. Ecol. Indic. 2020, 120, 106940. [Google Scholar] [CrossRef]
- Ouyang, W.; Ju, X.; Gao, X.; Hao, F.; Gao, B. Ecological security assessment of agricultural development watershed considering non-point source pollution. China Environ. Sci. 2018, 38, 1194–1200. [Google Scholar]
- Qian, C.; Chen, W.; Gong, B.; Wang, L.-F.; Yu, H.-Q. Diagnosis of the unexpected fluorescent contaminants in quantifying dissolved organic matter using excitation-emission matrix fluorescence spectroscopy. Water Res. 2019, 163, 114873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Ni, J.; Xie, D. Adoption behavior of cleaner production techniques to control agricultural non-point source pollution: A case study in the Three Gorges Reservoir Area. J. Clean. Prod. 2019, 223, 897–906. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop Residue Burning in India: Policy Challenges and Potential Solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, H.; Withers, P.; Bowes, M.; Palmer-Felgate, E.; Harper, D.; Wasiak, K.; Wasiak, P.; Hodgkinson, R.; Bates, A.; Stoate, C.; et al. Streamwater phosphorus and nitrogen across a gradient in rural–agricultural land use intensity. Agric. Ecosyst. Environ. 2010, 135, 238–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhou, Y.; Zhang, L.; Yao, X.; Shi, K.; Jeppesen, E.; Yu, Q.; Zhu, W. Chromophoric dissolved organic matter in inland waters: Present knowledge and future challenges. Sci. Total. Environ. 2020, 759, 143550. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Aiken, G.R.; Gilmour, C.C.; Krabbenhoft, D.P.; Orem, W. Dissolved organic matter in the Florida Everglades: Implications for ecosystem restoration. Crit. Rev. Environ. Sci. Technol. 2011, 41, 217–248. [Google Scholar] [CrossRef]
- Spencer, R.G.; Butler, K.D.; Aiken, G.R. Dissolved organic carbon and chromophoric dissolved organic matter properties of rivers in the USA. J. Geophys. Res. Biogeosciences 2012, 117, G03001. [Google Scholar] [CrossRef]
- Medeiros, P.M.; Seidel, M.; Ward, N.D.; Carpenter, E.J.; Gomes, H.R.; Niggemann, J.; Krusche, A.V.; Richey, J.E.; Yager, P.L.; Dittmar, T. Fate of the Amazon River dissolved organic matter in the tropical Atlantic Ocean. Glob. Biogeochem. Cycles 2015, 29, 677–690. [Google Scholar] [CrossRef]
- Williams, C.J.; Frost, P.C.; Morales-Williams, A.M.; Larson, J.H.; Richardson, W.B.; Chiandet, A.S.; Xenopoulos, M.A. Human activities cause distinct dissolved organic matter composition across freshwater ecosystems. Glob. Chang. Biol. 2016, 22, 613–626. [Google Scholar] [CrossRef]
- Yamashita, Y.; Jaffé, R.; Maie, N.; Tanoue, E. Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC). Limnol. Oceanogr. 2008, 53, 1900–1908. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Mao, R.; Li, S. Hydrological seasonality largely contributes to riverine dissolved organic matter chemical composition: Insights from EEM-PARAFAC and optical indicators. J. Hydrol. 2021, 595, 125993. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Che, X.; Li, C.; Cui, Z.; Su, R.; Qu, K. Indirect photodegradation of sulfadiazine in the presence of DOM: Effects of DOM components and main seawater constituents. Environ. Pollut. 2021, 268, 115689. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Cao, C.; Lin, M.; Qiu, Q.; Xu, Y.; Ren, Y. Compositional variety of dissolved organic matter and its correlation with water quality in peri-urban and urban river watersheds. Ecol. Indic. 2019, 104, 459–469. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Yang, L.; Qiu, Q.; Lin, M.; Cao, C.; Li, X. Seasonal variation and ecological risk assessment of dissolved organic matter in a peri-urban critical zone observatory watershed. Sci. Total Environ. 2020, 707, 136093. [Google Scholar] [CrossRef]
- Baker, A.; Ward, D.; Lieten, S.H.; Periera, R.; Simpson, E.C.; Slater, M. Measurement of protein-like fluorescence in river and waste water using a handheld spectrophotometer. Water Res. 2004, 38, 2934–2938. [Google Scholar] [CrossRef]
- DeVilbiss, S.E.; Zhou, Z.; Klump, J.V.; Guo, L. Spatiotemporal variations in the abundance and composition of bulk and chromophoric dissolved organic matter in seasonally hypoxia-influenced Green Bay, Lake Michigan, USA. Sci. Total Environ. 2016, 565, 742–757. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, F.; Kung, H.-T.; Ghulam, A.; Trumbo, A.L.; Yang, J.; Ren, Y.; Jing, Y. Evaluation and estimation of surface water quality in an arid region based on EEM-PARAFAC and 3D fluorescence spectral index: A case study of the Ebinur Lake Watershed, China. Catena 2017, 155, 62–74. [Google Scholar] [CrossRef]
- Lintern, A.; Webb, J.; Ryu, D.; Liu, S.; Bende-Michl, U.; Waters, D.; Leahy, P.; Wilson, P.; Western, A. Key factors influencing differences in stream water quality across space. WIREs Water 2018, 5, e1260. [Google Scholar] [CrossRef] [Green Version]
- Masese, F.O.; Salcedo-Borda, J.S.; Gettel, G.M.; Irvine, K.; McClain, M.E. Influence of catchment land use and seasonality on dissolved organic matter composition and ecosystem metabolism in headwater streams of a Kenyan river. Biogeochemistry 2017, 132, 1–22. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr-Met. 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Walker, S.A.; Amon, R.M.; Stedmon, C.A. Variations in high-latitude riverine fluorescent dissolved organic matter: A comparison of large Arctic rivers. J. Geophys. Res. Biogeosciences 2013, 118, 1689–1702. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor–an online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Chen, X.; Wang, D.; Liang, J.; Bai, W.; Zhang, C.; Wang, Q.; Wei, S. Dynamics of dissolved organic matter (DOM) in a typical inland lake of the Three Gorges Reservoir area: Fluorescent properties and their implications for dissolved mercury species. J. Environ. Manag. 2018, 206, 418–429. [Google Scholar] [CrossRef]
- Li, S.; Bush, R.T.; Santos, I.R.; Zhang, Q.; Song, K.; Mao, R.; Wen, Z.; Lu, X.X. Large greenhouse gases emissions from China’s lakes and reservoirs. Water Res. 2018, 147, 13–24. [Google Scholar] [CrossRef]
- Barakat, A.; El Baghdadi, M.; Rais, J.; Aghezzaf, B.; Slassi, M. Assessment of spatial and seasonal water quality variation of Oum Er Rbia River (Morocco) using multivariate statistical techniques. Int. Soil Water Conserv. Res. 2016, 4, 284–292. [Google Scholar] [CrossRef]
- Heibati, M.; Stedmon, C.A.; Stenroth, K.; Rauch, S.; Toljander, J.; Säve-Söderbergh, M.; Murphy, K.R. Assessment of drinking water quality at the tap using fluorescence spectroscopy. Water Res. 2017, 125, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ciputra, S.; Antony, A.; Phillips, R.; Richardson, D.; Leslie, G. Comparison of treatment options for removal of recalcitrant dissolved organic matter from paper mill effluent. Chemosphere 2010, 81, 86–91. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnol. Oceanogr. 2005, 50, 1415–1426. [Google Scholar] [CrossRef]
- Williams, C.J.; Yamashita, Y.; Wilson, H.F.; Jaffé, R.; Xenopoulos, M.A. Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol. Oceanogr. 2010, 55, 1159–1171. [Google Scholar]
- Lapierre, J.F.; Frenette, J.J. Effects of macrophytes and terrestrial inputs on fluorescent dissolved organic matter in a large river system. Aquat. Sci. 2009, 71, 15–24. [Google Scholar]
- Dey, S.; Botta, S.; Kallam, R.; Angadala, R.; Andugala, J. Seasonal variation in water quality parameters of Gudlavalleru Engineering College pond. Curr. Res. Green Sustain. Chem. 2021, 4, 100058. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Tranvik, L.; Kronberg, L.; Slätis, T.; Martinsen, W. Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea. Mar. Chem. 2007, 104, 227–240. [Google Scholar]
- Holbrook, R.D.; Yen, J.H.; Grizzard, T.J. Characterizing natural organic material from the Occoquan Watershed (Northern Virginia, US) using fluorescence spectroscopy and PARAFAC. Sci. Total Environ. 2006, 361, 249–266. [Google Scholar]
- Chen, M.; Price, R.M.; Yamashita, Y.; Jaffé, R. Comparative study of dissolved organic matter from groundwater and surface water in the Florida coastal Everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Appl. Geochem. 2010, 25, 872–880. [Google Scholar] [CrossRef]
- Mayorga, E.; Aufdenkampe, A.K.; Masiello, C.A.; Krusche, A.V.; Hedges, J.I.; Quay, P.D.; Richey, J.E.; Brown, T.A. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature 2005, 436, 538–541. [Google Scholar]
- Yang, L.; Hong, H.; Guo, W.; Huang, J.; Li, Q.; Yu, X. Effects of changing land use on dissolved organic matter in a subtropical river watershed, southeast China. Reg. Environ. Chang. 2011, 12, 145–151. [Google Scholar] [CrossRef]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Zeng, Z.; Zheng, P.; Ding, A.; Zhang, M.; Abbas, G.; Li, W. Source analysis of organic matter in swine wastewater after anaerobic digestion with EEM-PARAFAC. Environ. Sci. Pollut. Res. 2017, 24, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Borisover, M.; Laor, Y.; Saadi, I.; Lado, M.; Bukhanovsky, N. Tracing organic footprints from industrial effluent discharge in recalcitrant riverine chromophoric dissolved organic matter. Water Air Soil Pollut. 2011, 222, 255–269. [Google Scholar] [CrossRef]
- Werner, B.J.; Lechtenfeld, O.J.; Musolff, A.; de Rooij, G.H.; Yang, J.; Gründling, R.; Fleckenstein, J.H. Small-scale topography explains patterns and dynamics of dissolved organic carbon exports from the riparian zone of a temperate, forested catchment. Hydrol. Earth Syst. Sci. Discuss. 2021, 25, 6067–6086. [Google Scholar] [CrossRef]
- Jiang, M.; Sheng, Y.; Tian, C.; Li, C.; Liu, Q.; Li, Z. Feasibility of source identification by DOM fingerprinting in marine pollution events. Mar. Pollut. Bull. 2021, 173, 113060. [Google Scholar] [CrossRef]
- Jiang, T.; Skyllberg, U.; Björn, E.; Green, N.W.; Tang, J.; Wang, D.; Gao, J.; Li, C. Characteristics of dissolved organic matter (DOM) and relationship with dissolved mercury in Xiaoqing River-Laizhou Bay estuary, Bohai Sea, China. Environ. Pollut. 2017, 223, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wu, Y.; Wang, F.; Xu, D.; Liu, S.; Zhou, M. Hotspot of Organic Carbon Export Driven by Mesoscale Eddies in the Slope Region of the Northern South China Sea. Front. Mar. Sci. 2020, 7, 444. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, B.; Song, Y.; Qin, Y. Correlation between molecular absorption spectral slope ratios and fluorescence humification indices in characterizing CDOM. Aquat. Sci. 2010, 73, 103–112. [Google Scholar] [CrossRef]
- Shi, W.; Zhuang, W.-E.; Hur, J.; Yang, L. Monitoring dissolved organic matter in wastewater and drinking water treatments using spectroscopic analysis and ultra-high resolution mass spectrometry. Water Res. 2020, 188, 116406. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Ward, D.; Reynolds, D.M.; Brunsdon, C.; Carliell-Marquet, C.; Browning, S. Can fluorescence spec-trometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 2008, 391, 149–158. [Google Scholar] [CrossRef]
- Aiken, G.R.; Spencer, R.G.; Striegl, R.G.; Schuster, P.F.; Raymond, P.A. Influences of glacier melt and permafrost thaw on the age of dissolved organic carbon in the Yukon River basin. Glob. Biogeochem. Cycles 2014, 28, 525–537. [Google Scholar] [CrossRef]






| Model | Refence Model λEx/λEm (Ref.) | Probable Fluorescence Peak | Components, EEM Contours, Spectral Loadings, and Location | Description | |
|---|---|---|---|---|---|
| C1: 272/354 | C7: 279/348 C3: 273/352 C8: 270/360 | T |  |  | Protein-like free tryptophan derived from autochthonous processes |
| C2: 272/302 | C3: 266/302 C5: 277/326 C5: 274/318 | B |  |  | Protein-like free tyrosine-like moieties attributed to microbial origin |
| C3: 308/393 | C2: 241(307)/398 C2: 302/400 | D |  |  | Humic-like, Fulvic-like, similar to semiquinone molecules |
| C4: 263/442 | C2: 258/470 | C |  |  | Humic-like, of terrestrial origin, poor contribution of macrophytes, allochthonous |
| C1: [38,39], C2: [33,40], C3 [35,36], and C4: [33] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, G.; Zhang, B.; Tian, B.; Yang, R.; Baker, A.; Wu, P.; He, S. Characterization of Dissolved Organic Matter from Agricultural and Livestock Effluents: Implications for Water Quality Monitoring. Int. J. Environ. Res. Public Health 2023, 20, 5121. https://doi.org/10.3390/ijerph20065121
Qi G, Zhang B, Tian B, Yang R, Baker A, Wu P, He S. Characterization of Dissolved Organic Matter from Agricultural and Livestock Effluents: Implications for Water Quality Monitoring. International Journal of Environmental Research and Public Health. 2023; 20(6):5121. https://doi.org/10.3390/ijerph20065121
Chicago/Turabian StyleQi, Guizhi, Borui Zhang, Biao Tian, Rui Yang, Andy Baker, Pan Wu, and Shouyang He. 2023. "Characterization of Dissolved Organic Matter from Agricultural and Livestock Effluents: Implications for Water Quality Monitoring" International Journal of Environmental Research and Public Health 20, no. 6: 5121. https://doi.org/10.3390/ijerph20065121