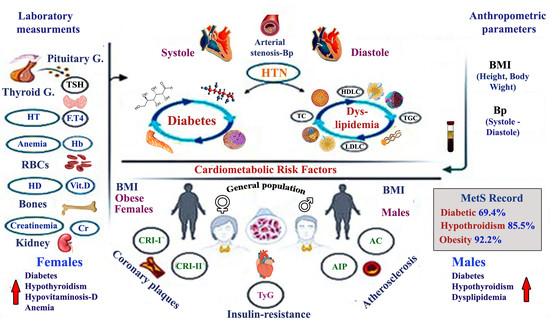
Metabolic Syndrome and Cardiometabolic Risk Factors in the Mixed Hypercholesterolemic Populations with Respect to Gender, Age, and Obesity in Asir, Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population and Studied Parameters
2.2. Laboratory Assays, Data Curation, and Reference Ranges
2.3. Body Mass Index (BMI) and Blood Pressure (Bp: S/D)
2.4. Statistical Analyses
3. Results
3.1. Metabolic Profiles of the General Population Concerning Gender
3.2. Metabolic Syndrome (MetS) According to Age with Respect to Gender in the General Population
3.3. MetS According to BMI in the General Population
3.4. Cardiometabolic Risk Factors and Insulin-Resistance Marker
3.5. Correlations and Hierarchical Clustering of the Lipid Profiles and Cardiometabolic Risk Factors
3.6. Prevalence of Cardiac Risk and MetS in the Mixed-Hypercholesterolemic (HC) Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ruotolo, G.; Howard, B.V. Dyslipidemia of the metabolic syndrome. Curr. Cardiol. Rep. 2002, 4, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic Syndrome: Connecting and Reconciling Cardiovascular and Diabetes Worlds. J. Amer Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Hansen, B.; Smith, S.C., Jr.; Cleeman, J.I.; Kahn, R.A. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e19–e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaven, G.M. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. Jama 2002, 287, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.J. Abnormalities of coagulation, platelet function, and fibrinolysis associated with syndromes of insulin resistance. Coronary Artery Dis. 2005, 16, 473–476. [Google Scholar] [CrossRef]
- Al-Lawati, J.A.; Mohammed, A.J.; Al-Hinai, H.Q.; Jousilahti, P.P. Prevalence of the metabolic syndrome among Omani adults. Diabetes Care. 2003, 26, 1781–1785. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Razig, S.A. The prevalence of the metabolic syndrome among the multiethnic population of the United Arab Emirates: A report of a national survey. Metab. Syndr. Relat. Disord. 2008, 6, 177–186. [Google Scholar] [CrossRef]
- Al-Nozha, M.; Al-Khadra, A.; Arafah, M.R.; Al-Maatouq, M.A.; Khalil, M.Z.; Khan, N.B.; Al-Mazrou, Y.Y.; Al-Marzouki, K.; Al-Harthi, S.S.; Abdullah, M.; et al. Metabolic syndrome in Saudi Arabia. Saudi Med. J. 2005, 26, 1918–1925. [Google Scholar]
- Al-Rubeaan, K.; Bawazeer, N.; Al Farsi, Y.; Youssef, A.M.; Al-Yahya, A.A.; AlQumaidi, H.; Al-Malki, B.M.; Naji, K.A.; Al-Shehri, K.; Al Rumaih, F.I. Prevalence of metabolic syndrome in Saudi Arabia—A cross sectional study. BMC Endocr. Disord. 2018, 18, 3–9. [Google Scholar] [CrossRef]
- Mohan, V.; Deepa, M. The metabolic syndrome in developing countries. Diabetes Voice 2006, 51, 80151. [Google Scholar]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, lung, and blood institute/American Heart Ass. conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Butte, N.F.; Comuzzie, A.G.; Cole, S.A.; Mehta, N.R.; Cai, G.; Tejero, M.; Bastarrachea, R.; Smith, E.O.B. Quantitative Genetic Analysis of the Metabolic Syndrome in Hispanic Children. Pediatric Res. 2005, 58, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsen, B.; Lahti, K.; Nissen, M.; Taskinen, M.-R.; Groop, L.J.D.C. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.C., Jr.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Cerqueira, M.D.; Dracup, K.; Fuster, V.; Gotto, A.; Grundy, S.M.; Miller, N.H.; et al. AHA/ACC Guidelines for Preventing Heart Attack and Death in Patients With Atherosclerotic Cardiovascular Disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J. Am. Coll. Cardiol. 2001, 104, 1581–1583. [Google Scholar] [CrossRef]
- Heinz, F.; Beushausen, T.W. A new enzymatic method for the determination of glucose. J. Clin. Chem. Clin. Biochem. 1981, 19, 977–978. [Google Scholar] [CrossRef] [Green Version]
- Penttilä, I.; Penttilä, K.; Holm, P.; Laitinen, H.; Ranta, P.; Törrönen, J.; Rauramaa, R. Methods, units and quality requirements for the analysis of haemoglobin A1c in diabetes mellitus. World J. Methodol. 2016, 26, 133–142. [Google Scholar] [CrossRef]
- Bea, A.M.; Franco-Marín, E.; Marco-Benedí, V.; Jarauta, E.; Gracia-Rubio, I.; Cenarro, A.; Civeira, F.; Lamiquiz-Moneo, I. ANGPTL3 gene variants in subjects with familial combined hyperlipidemia. Sci. Rep. 2021, 11, 7002. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of low−density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, L.M.; Van Uytfanghe, K.; Poppe, K.; Velkeniers, B. Determination of free thyroid hormones. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.M.; Soldevila, B.; Velasco, I.; Moreno-Flores, F.; Ferrer, L.; Pérez-Montes de Oca, A.; Santillán, C.; Muñoz, C.; Ballesta, S.; Canal, C.; et al. Reference Intervals of Thyroid Function Tests Assessed by Immunoassay and Mass Spectrometry in Healthy Pregnant Women Living in Catalonia. J. Clin. Med. 2021, 31, 2444. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, E.; Björkhem-Bergman, L. Comparison of Creatinine and Cystatin C to Estimate Renal Function in Geriatric and Frail Patients. Life 2022, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Glycemic Targets: Standards of Medical Care in Diabetes 2020. Diabetes Care 2020, 43, S66–S76. [CrossRef]
- World Health Organization. Part 1: Diagnosis and classification of diabetes mellitus. In Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications; World Health Organization: Geneva, Switzerland, 1999. Available online: https://apps.who.int/iris/handle/10665/66040 (accessed on 17 June 2012).
- Aslesh, O.P.; Jayasree, A.K.; Karunakaran, U.; Venugopalan, A.K.; Divakaran, B.; Mayamol, T.R.; Sunil, C.B.; Minimol, K.J.; Shalini, K.; Mallar, G.; et al. Prevalence of hypercholesterolaemia among adults aged over 30 years in a rural area of north Kerala, India: A cross-sectional study. WHO South East Asia J. Public Health 2016, 5, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Third Report of the Expert Panel on Detection. Evaluation, and Treatment of the High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive Summary. Available online: http://wwwnhlbinihgov/guidelines/cholesterol/atp3_rpthtm. (accessed on 9 October 2010).
- Miller, M.; Stone, N.J.; Ballantyne, C.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; Lennie, T.A.; et al. Triglycerides and Cardiovascular Disease. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [Green Version]
- National Cholesterol Education Program (NCEP). Expert panel, Third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation and treatment of high cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Normal Serum Vitamin D Levels. N. Engl. J. Med. 2005, 352, 515–516. [Google Scholar] [CrossRef]
- Sheehan, M.T. Biochemical Testing of the Thyroid: TSH is the Best and, Oftentimes, Only Test Needed—A Review for Primary Care. Clin. Med. Res. 2016, 14, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heil, W.; Ehrhardt, V. Reference Intervals for Adults and Children 2008, 9th ed.; Roche Diagnostics Ltd.: Rotkreuz, Switzerland, 2009; Volume V9.1. [Google Scholar]
- Mirjanic-Azaric, B.; Avram, S.; Stojakovic-Jelisavac, T.; Stojanovic, D.; Petkovic, M.; Bogavac-Stanojevic, N.; Ignjatovic, S.; Stojanov, M. Direct Estimation of Reference Intervals for Thyroid Parameters in the Republic of Srpska. J. Med. Biochem. 2017, 36, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Batte, A.; Berrens, Z.; Murphy, K.; Mufumba, I.; Sarangam, M.L.; Hawkes, M.T.; Conroy, A.L. Malaria-Associated Acute Kidney Injury in African Children: Prevalence, Pathophysiology, Impact, and Management Challenges. Int. J. Nephrol. Renovas. Dis. 2021, 14, 235–253. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am. J. Kidney Dis. 2006, 47, S1–S146. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cheng, H.M.; Chen, C.H.; Sung, S.H.; Moslehpour, M.; Hahn, J.O.; Mukkamala, R. Patient-Specific Oscillometric Blood Pressure Measurement. IEEE Trans. Biomed. Eng. 2016, 63, 1220–1228. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Shim, Y.S.; Lee, H.S.; Hwang, I.T.; Hwang, J.S. A population-based study of TyG index distribution and its relationship to cardiometabolic risk factors in children and adolescents. Sci. Rep. 2021, 11, 23660. [Google Scholar] [CrossRef]
- Alzaheb, R.A.; Altemani, A.H. Prevalence and Associated Factors of Dyslipidemia Among Adults with Type 2 Diabetes Mellitus in Saudi Arabia. Diabetes Metab. Syndr Obes. 2020, 13, 4033–4040. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Mehta, R.K.; Koirala, P.; Mallick, R.L.; Parajuli, S.; Jha, R. Dyslipidemia in Patients with Type 2 Diabetes Mellitus in a Tertiary Care Centre: A Descriptive Cross-sectional Study. JNMA J. Nepal. Med. Assoc. 2021, 59, 305–309. [Google Scholar] [CrossRef]
- Yi, Y.; An, J. Sex Differences in Risk Factors for Metabolic Syndrome in the Korean Population. Int. J. Environ. Res. Public. Health 2020, 17, 9513. [Google Scholar] [CrossRef] [PubMed]
- Beigh, S.H.; Jain, S. Prevalence of metabolic syndrome and gender differences. Bioinformatics 2012, 8, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Al-Musa, H.M. Screening for dyslipidemia among Saudi adults attending a primary health care in Saudi Arabia. KKU J. Med. Sci. 2016, 1, 12–19. Available online: http://www.kkujhs.org (accessed on 8 August 2020).
- Pradhan, A.; Bhandari, M.; Vishwakarma, P.; Sethi, R. Triglycerides and Cardiovascular Outcomes-Can We REDUCE-IT ? Int. J. Angiol. 2020, 29, 2–11. [Google Scholar] [CrossRef]
- Noroozi, M.; Rastegari, Z.; Paknahad, Z. Type of body fat distribution in postmenopausal women and its related factors. Iran J. Nurs. Midwifery Res. 2010, 15, 27–31. [Google Scholar]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 136–147. [Google Scholar] [CrossRef]
- Neels, J.G.; Olefsky, J.M. Inflamed fat: What starts the fire? J. Clin. Invest. 2006, 116, 33–35. [Google Scholar] [CrossRef] [Green Version]
- Koleva, D.I.; Orbetzova, M.M.; Atanassova, P.K. Adipose tissue hormones and appetite and body weight regulators in insulin resistance. Folia Med. 2013, 55, 25–32. [Google Scholar] [CrossRef]
- Ogbera, A.O. Prevalence and gender distribution of the metabolic syndrome. Diabetol. Metab. Syndr. 2010, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Vishram, J.K.K.; Borglykke, A.; Andreasen, A.H.; Jeppesen, J.; Ibsen, H.; Jørgensen, T.; Palmieri, L.; Giampaoli, S.; Donfrancesco, C.; Kee, F.; et al. Impact of Age and Gender on the Prevalence and Prognostic Importance of the Metabolic Syndrome and Its Components in Europeans. The MORGAM Prospective Cohort Project. PLoS ONE 2014, 9, e107294. [Google Scholar] [CrossRef] [Green Version]
- Assmann, G.; Guerra, R.; Fox, G.; Cullen, P.; Schulte, H.; Willett, D.; Grundy, S.M. Harmonizing the Definition of the Metabolic Syndrome: Comparison of the Criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European Populations. Amer. J. Cardiol. 2007, 99, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Smith, G.D.; Ebrahim, S. Does the new International Diabetes Federation definition of the metabolic syndrome predict CHD any more strongly than older definitions? Findings from the British Women’s Heart and Health Study. Diabetogia 2006, 49, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Bouloukos, V.I.; Pehlivanidis, A.N.; Papageorgiou, A.A.; Dionysopoulou, S.G.; Symeonidis, A.N.; Petridis, D.I.; Kapousouzi, M.I.; Satsoglou, E.A.; Mikhailidis, D.P. The prevalence of the metabolic syndrome in Greece: The MetS-Greece Multicentre Study. Diabetes Obes. Metabol. 2005, 7, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Balkau, B.; Charles, M.-A. Comment on the provisional report from the WHO consultation. Diabetic Med. 1999, 16, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Mandoura, N.; Al-Raddadi, R.; Abdulrashid, O.; Shah, H.B.U.; Kassar, S.M.; Adel Hawari, A.R.; Jahhaf, J.M. Factors Associated with Consuming Junk Food among Saudi Adults in Jeddah City. Cureus 2017, 9, e2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, S.A.; Durgampudi, P.K. Factors associated with tobacco smoking among Saudi college students: A systematic review. Tob. Prev. Cessat. 2020, 12, 36. [Google Scholar] [CrossRef]
- Sarwar, N.; Sandhu, M.S.; Ricketts, S.L.; Butterworth, A.S.; Di Angelantonio, E.; Boekholdt, S.M.; Ouwehand, W.; Watkins, H.; Samani, N.J. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet 2010, 375, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.A.; Ko, Y.L.; Chang, C.J.; Teng, M.S.; Wu, S.; Hu, C.F. Apolipoprotein A5 gene -1131T/C polymorphism is associated with the risk of metabolic syndrome in ethnic Chinese in Taiwan. Clin. Chem. Lab. Med. 2008, 46, 1714–1719. [Google Scholar] [CrossRef]
- Fezeu, L.; Balkau, B.; Kengne, A.P.; Sobngwi, E.; Mbanya, J.C. Metabolic syndrome in a sub-Saharan African setting: Central obesity may be the key determinant. Atherosclerosis 2007, 193, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahonen, S.-M.; Liikanen, E. Development and challenges of a new academic discipline, radiography science. Eur. J. Radio. 2009, 1, 81–84. [Google Scholar] [CrossRef]
- Langenberg, C.; Sharp, S.J.; Schulze, M.B.; Rolandsson, O.; Overvad, K.; Forouhi, N.G.; Spranger, J.; Drogan, D.; Huerta, J.M.; Arriola, L.; et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: The EPIC-InterAct case-cohort study. PLoS Med. 2012, 9, e1001230. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. In Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014.
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Mattace Raso, F.U.; Muiesan, M.L.; Ryliškytė, L.; Rietzschel, E.; et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prevent. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef]
- ÄRnlöV, J.; Ingelsson, E.; SundströM, J.; Lind, L. Impact of Body Mass Index and the Metabolic Syndrome on the Risk of Cardiovascular Disease and Death in Middle-Aged Men. Circulation 2010, 121, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Meigs, J.B.; Wilson, P.W.F.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D’Agostino, R.B. Body Mass Index, Metabolic Syndrome, and Risk of Type 2 Diabetes or Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.F.; Börgermann, J. The role of vitamin D in dyslipidemia and cardiovascular disease. Curr. Pharm. Des. 2011, 17, 933–942. [Google Scholar] [CrossRef]
- Weissglas-Volkov, D.; Pajukanta, P. Genetic causes of high and low serum HDL-cholesterol. J. Lipid Res. 2010, 51, 2032–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.N.; Alkhenizan, A.H.; El Shaker, M.; Raef, H.; Gabr, A. Increasing trends and significance of hypovitaminosis D: A population-based study in the Kingdom of Saudi Arabia. Arch. Osteoporos. 2014, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Buyukuslu, N.; Esin, K.; Hizli, H.; Sunal, N.; Yigit, P.; Garipagaoglu, M. Clothing preference affects vitamin D status of young women. Nutr. Res. 2014, 34, 688–693. [Google Scholar] [CrossRef] [PubMed]
- AlFaris, N.A.; AlKehayez, N.M.; AlMushawah, F.I.; AlNaeem, A.N.; AlAmri, N.D.; AlMudawah, E.S. Vitamin D Deficiency and Associated Risk Factors in Women from Riyadh, Saudi Arabia. Sci. Rep. 2019, 9, 20371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleva, D.; Gateva, P.; Orbetzova, M.; Atanassova, I.; Nikolova, J. Atherogenic Index of Plasma, Castelli Risk Indexes and Leptin/Adiponectin Ratio in Women with Metabolic Syndrome. Int. J. Pharmaceut. Med. Res. 2015, 3, 12–18. [Google Scholar]
- Nwagha, U.I.; Ikekpeazu, E.J.; Ejezie, F.E.; Neboh, E.E.; Maduka, I.C. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. African Health Sci. 2010, 10, 248–252. [Google Scholar]
- Li, Y.W.; Kao, T.W.; Chang, P.K.; Chen, W.L.; Wu, L.W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: A 9-year longitudinal study. Sci. Rep. 2021, 11, 9900. [Google Scholar] [CrossRef]
- Onat, A.; Can, G.; Kaya, H.; Hergenç, G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J. Clin. Lipidol. 2010, 4, 89–98. [Google Scholar] [CrossRef]
- Ginsberg, H.N. New perspectives on atherogenesis: Role of abnormal triglyceride-rich lipoprotein metabolism. Circulation 2002, 106, 2137–2142. [Google Scholar] [CrossRef] [Green Version]
- Rye, K.A.; Bursill, C.A.; Lambert, G.; Tabet, F.; Barter, P.J. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 2009, 50, S195–S200. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Fu, J.; Koonen, D.P.; Kuivenhoven, J.A.; Snieder, H.; Hofker, M.H. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis 2014, 233, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Kelley, D.E. Skeletal muscle triglyceride: Marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Current Diabetes Rep. 2002, 2, 216–222. [Google Scholar] [CrossRef] [PubMed]




| MetS-Related Criteria | † Parameters | Characteristics of the General Population According to Gender | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Profile | % | † Males | N | % | † Females | N | % | ||||
| Body Mass Index–BMI (kg/m2) | 33.7 ± 0.61 | 92.2 | 27.73 ± 0.27 | 3/5 | 60.0 | 33.27 ± 4.26 | 3/4 | 75.0 | 0.26 | ||
| Diabetes T2DM | † A1c ≥ 6.4 (%) | 9.2 ± 0.11 | 69.4 | 9.51 ± 0.22 | 86/144 | 59.7 | 9.13 ± 0.21 | 127/196 | 64.8 | 0.11 | |
| † FSG ≥ 125 (mg/dL) | 212 ± 5.1 | 53.2 | 229.1 ± 12.3 | 64/133 | 48.1 | 210.9 ± 8.40 | 85/190 | 44.7 | 0.10 | ||
| Dyslipidemia | † TC: ≥ 240 (mg/dL) | 280.0 ± 7.1 | 5.20 | 293.8 ± 16.8 | 10/179 | 5.60 | 279.0 ± 9.36 | 10/261 | 3.83 | 0.23 | |
| † TGC: ≥ 200 (mg/dL) | 274.5 ± 6.3 | 21.2 | 280.7 ± 9.92 | 50/177 | 28.3 | 256.2 ± 10.7 | 42/247 | 17.0 | 0.08 | ||
| HDLC mg/dL | L.: <40 | 32.6 ± 0.4 | 37.7 | 32.2 ± 0.81 | 66/135 | 48.9 | 33.8 ± 0.91 | 55/202 | 27.2 | 0.19 | |
| H.: >59 | 77.2 ± 3.9 | 9.10 | 68.0 ± 3.34 | 6/135 | 4.40 | 69.8 ± 2.53 | 22/202 | 10.9 | 0.72 | ||
| LDLC mg/dL | L.: <100 | 73.4 ± 1.3 | 53.3 | 68.6 ± 2.65 | 66/126 | 52.4 | 72.0 ± 2.20 | 89/179 | 49.7 | 0.32 | |
| H.: >129 | 156.1 ± 3.1 | 20.9 | 157.4 ± 5.79 | 37/126 | 29.4 | 151.0 ± 3.10 | 37/179 | 20.7 | 0.32 | ||
| Hypovitaminosis-D (Vit. D: ng/mL) | L.: <20 | 13.49 ± 0.3 | 56.2 | 13.9 ± 0.74 | 77/144 | 53.5 | 13.16 ± 0.36 | 138/250 | 55.1 | 0.16 | |
| H.: >50 | 53.5 ± 0.47 | 2.26 | 53.4 ± 0.47 | 7/144 | 4.86 | 53.70 ± 1.11 | 4/250 | 1.60 | 0.39 | ||
| Hypothyroidism (HT) | TSH uIU/mL | L.: <0.4 | 0.24 ± 0.11 | 3.30 | 0.11 ± 0.04 | 8/174 | 4.60 | 0.15 ± 0.03 | 10/321 | 3.12 | 0.21 |
| H.: >5.0 | 7.48 ± 0.23 | 19.9 | 7.50 ± 0.54 | 20/174 | 11.50 | 7.75 ± 0.31 | 77/321 | 23.9 | 0.36 | ||
| F.T4 pmol/L | L.: <12.0 | 8.79 ± 0.09 | 85.5 | 8.81 ± 0.25 | 71/91 | 78.0 | 8.71 ± 0.12 | 186/212 | 87.7 | 0.34 | |
| H.: ≥20.0 | 22.2 ± 0.00 | 0.26 | 22.2 ± 0.00 | 1/91 | 1.10 | ---- | ---- | ---- | ---- | ||
| Anemia | † Hb: <13.5 M-12.0 F. (g/dL) | 10.9 ± 0.12 | 24.9 | 11.9 ± 0.37 | 17/190 | 8.95 | 10.5 ± 0.12 * | 101/280 | 36.1 | <0.0001 | |
| Creatinemia | Cr mg/dL | L. <0.7 M.–0.5 F. | 0.57 ± 0.11 | 10.2 | 0.58 ± 0.02 | 18/183 | 9.80 | 0.41 ± 0.01 * | 30/290 | 10.4 | |
| H. >1.2 M.–1.0 F. | 1.44 ± 0.05 | 10.4 | 1.70 ± 0.17 | 20/183 | 10.9 | 1.30 ± 0.07 * | 29/290 | 10.0 | 0.0185 | ||
| Hypertension (HTN) | † Systole: ≥ 140 mm Hg | 152.9 ± 1.6 | 25.5 | 148.0 ± 3.52 | 9/27 | 33.3 | 143.6 ± 1.1 | 13/57 | 22.8 | 0.09 | |
| † Diastole: ≥ 90 mm Hg | 75.0 ± 1.39 | 25.5 | 80.1 ± 3.26 | 9/27 | 33.3 | 76.2 ± 1.9 | 13/57 | 22.8 | 0.14 | ||
| Blood Pressure | 153/75 | 26.0 | 148/80 | 9/27 | 33.3 | 144/76 | 13/57 | 22.8 | -- | ||
| Gender | Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | <40 Y. | N | ≥40 Y. | N. | p Value | <40 Y. | N | ≥40 Y. | N. | p Value |
| 31.9 ± 1.5 | 14 | 61.8 ± 2.30 * | 20 | <0.0001 | 31.9 ± 0.83 | 36 | 59.6 ± 2.70 * | 33 | <0.0001 | |
| A1C (%) | 5.5 ± 0.09 | 9 | † 8.6 ± 1.20 * | 8 | 0.015 | 6.00 ± 0.00 | 2 | † 6.97 ± 0.85 | 15 | --- |
| FSG (mg/dL) | 102.7 ± 1.1 | 5 | † 145.3 ± 29.4 | 6 | 0.223 | 87.4 ± 3.56 | 10 | † 139.1 ± 44.1 | 6 | 0.147 |
| TC (mg/dL) | 183.2 ± 10.8 | 9 | 167.2 ± 16.10 | 19 | 0.523 | 175.8 ± 9.6 | 11 | 179.7 ± 40.5 | 13 | 0.932 |
| TGC (mg/dL) | 113.1 ± 12.8 | 9 | 132.7 ± 22.40 | 19 | 0.569 | 95.3 ± 21.3 | 9 | 129.5 ± 43.9 | 11 | 0.522 |
| HDLC (mg/dL) | 41.6 ± 2.47 | 9 | † 37.4 ± 2.70 | 19 | 0.337 | 50.2 ± 4.03 | 11 | 47.3 ± 6.9 | 13 | 0.733 |
| LDLC (mg/dL) | 141.7 ± 4.6 | 7 | † 99.8 ± 18.60 | 16 | 0.158 | 104.2 ± 9.7 | 12 | 109.4 ± 28.9 | 16 | 0.882 |
| Vit.D (ng/mL) | 32.57 ± 7.1 | 6 | 25.0 ± 4.40 | 17 | 0.385 | † 16.13 ± 1.7 | 15 | 21.65 ± 2.6 | 27 | 0.146 |
| F. T4 (pmol/L) | † 9.96 ± 0.52 | 14 | 12.4 ± 1.65 | 20 | 0.238 | † 8.45 ± 0.25 | 35 | † 9.75 ± 0.70 | 33 | 0.078 |
| Hb (g/dL) | 14.89 ± 0.7 | 10 | 15.4 ± 0.60 | 16 | 0.592 | † 11.34 ± 0.4 | 28 | 12.75 ± 0.50 * | 29 | 0.033 |
| Cr (mg/dL) | 0.95 ± 0.05 | 12 | 1.04 ± 0.02 | 13 | 0.099 | 0.64 ± 0.03 | 19 | 0.83 ± 0.10 | 20 | 0.083 |
| Systole (mmHg) | 133.7 ± 3.1 | 12 | 134.9 ± 5.50 | 18 | 0.869 | 120.5 ± 2.5 | 29 | 129.3 ± 4.35 | 28 | 0.082 |
| † Parameters and MetS | Characteristics of Population According to BMI (kg/m2) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference Risk | O.W: 25–29.9 | N | % | Obese: ≥30 | N | % | |||
| Abnormal BMI (kg/m2) | >25 | 27.0 ± 0.2 | 57/153 | 37.3 | 37.0 ± 0.7 * | 90/153 | 58.8 | 0.0001 | |
| † High Systolic BP (mm Hg) | ≥140 | 157.1 ± 5.7 | 8/45 | 17.8 | 153.1 ± 1.9 | 35/83 | 42.2 | 0.204 NS | |
| † High Diastolic BP (mm Hg) | ≥90 | 90.0 ± 0.0 | 3/45 | 6.7 | NA | -- | -- | ---- | |
| Hypertension (S/D) | ≥140/90 | 164/86 | 7/45 | 15.6 | 154/72 | 34/83 | 41.1 | ---- | |
| Anemia–† Low Hb (g/dL) | <13.5 M–<12.0 F | 10.5 ± 0.50 | 7/50 | 14.0 | 11.1 ± 0.17 | 21/91 | 23.0 | 0.077 NS | |
| DM–† A1c (%) | >5.7 | 8.26 ± 0.24 | 42/45 | 93.3 | 9.01 ± 0.22 * | 88/90 | 97.8 | 0.019 | |
| DM–† FSG (mg/dL) | >125 | 180.8 ± 10.8 | 29/48 | 60.4 | 206.0 ± 9.1 | 62/88 | 70.5 | 0.051 NS | |
| HC–† High T.C (mg/dL) | ≥240 | 289.6 ± 26.8 | 4/47 | 8.50 | 270.2 ± 11.6 | 6/88 | 6.80 | 0.236 NS | |
| HC–Borderline (mg/dL) | 200–239 | 211.0 ± 4.8 | 8/9 | 88.9 | NA | 0 | 0.0 | ---- | |
| † High T.G.C (mg/dL) | >200 | 279.5 ± 14.1 | 12/44 | 27.3 | 293.3 ± 16.4 | 14/83 | 16.9 | 0.268 NS | |
| HDLC (mg/dL) | † Low | <40 | 41.0 ± 1.6 | 23/45 | 51.0 | 47.0 ± 2.4 * | 35/87 | 40.2 | 0.035 |
| † High | >59 | 64.4 ± 4.7 | 3/45 | 6.70 | 87.5 ± 10.6 | 12/87 | 13.8 | 0.155 NS | |
| LDLC (mg/dL) | † Low | <100 | 70.2 ± 3.1 | 22/40 | 55.0 | 80.8 ± 1.3 * | 48/80 | 60.0 | <0.0001 |
| † High | >129 | 165.2 ± 15.1 | 10/40 | 25.0 | 168.6 ± 4.1 | 6/80 | 7.50 | 0.434 | |
| Vit.D (ng/mL) | † Low | <20 | 13.5 ± 1.3 | 18/28 | 64.3 | 14.5 ± 0.7 | 40/61 | 65.6 | 0.232 |
| † High | >50 | 52.4 | 1/28 | 3.60 | 52.4 | 1/63 | 1.60 | ---- | |
| TSH (uIU/mL) | † Low | <0.3 | 0.20 ± 0.00 | 2/34 | 5.9 | NA | -- | -- | ---- |
| † High | >5.0 | 7.63 ± 1.18 | 7/34 | 20.6 | 6.49 ± 0.34 | 13/63 | 20.6 | 0.122 | |
| F.T4 (pmol/L) | † Low | <12 | 8.7 ± 0.3 | 24/29 | 82.8 | 9.2 ± 0.2 | 49/59 | 83.1 | 0.081 |
| † High | ≥20 | NA | -- | -- | NA | -- | -- | ---- | |
| Cr (mg/dL) | † Low | <0.7 M–<0.5 F | 0.57 ± 0.03 | 12/49 | 24.5 | 0.56 ± 0.01 | 38/89 | 42.7 | 0.342 |
| † High | >1.2 M–>1 F | 1.36 ± 0.06 | 12/49 | 24.5 | 1.37 ± 0.13 | 16/87 | 18.4 | 0.475 | |
| Metabolic Predictors | Serum Biomarkers in Different MetS’ Populations | Age | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dyslipidemia | DM | HT | HD | Anemia | High BP (S/D) | Creatinemia | ||||||||
| TC | TGC | Low HDLC | High LDLC | A1C | FSG | F.T4 | Vit. D | Low Hb | High Cr | Low Cr | ||||
| CRI-I | AUC | 0.864 * | 0.608 * | 0.805 * | 0.560 | 0.494 | 0.525 | 0.498 | 0.485 | 0.416 | 0.627 * | 0.526 | 0.583 | 0.604 |
| Cut-off | 4.99 | 4.83 | 4.31 | 4.37 | --- | 3.87 | --- | --- | --- | 3.71 | 3.67 | 3.18 | 2.59 | |
| SEN | 0.85 | 0.45 | 0.65 | 0.51 | --- | 0.52 | --- | --- | --- | 0.69 | 0.63 | 0.93 | 0.90 | |
| r | 0.569 * | 0.292 * | −0.592 * | 0.143 * | −0.035 | 0.078 | 0.064 | −0.005 | 0.125 * | −0.075 | 0.049 | 0.282 * | ||
| CRI-II | AUC | 0.735 * | 0.522 * | 0.725 * | 0.848 * | 0.408 | 0.470 | 0.486 | 0.454 | 0.478 | 0.609 * | 0.536 | 0.462 | 0.580 |
| Cut-off | 2.47 | 3.44 | 2.17 | 2.92 | --- | --- | --- | --- | --- | 2.37 | 4.19 | --- | 1.33 | |
| SEN | 0.77 | 0.26 | 0.76 | 0.76 | --- | --- | --- | --- | --- | 0.55 | 0.20 | --- | 0.82 | |
| r | 0.198 * | 0.115 * | −0.366 * | 0.754 * | −0.106 * | −0.009 | 0.087 | .024 | 0.085 | −0.056 | 0.132 * | 0.230 * | ||
| AIP | AUC | 0.865 * | 0.608 * | 0.807 * | 0.559 | 0.495 | 0.527 | 0.499 | 0.485 | 0.415 | 0.631 * | 0.524 | 0.583 | 0.605 |
| Cut-off | 0.71 | 0.69 | 0.64 | 0.65 | --- | 0.86 | --- | --- | --- | 0.57 | 0.61 | 0.51 | 0.42 | |
| SEN | 0.85 | 0.44 | 0.65 | 0.49 | --- | 0.53 | --- | --- | --- | 0.69 | 0.53 | 0.93 | 0.90 | |
| r | 0.563 * | 0.258 * | −0.729 * | 0.131 * | −0.022 | 0.079 | 0.064 | 0.040 | 0.053 | −0.074 | 0.052 | 0.280 * | ||
| AC | AUC | 0.864 * | 0.608 * | 0.805 * | 0.560 | 0.494 | 0.525 | 0.498 | 0.485 | 0.416 | 0.627 * | 0.526 | 0.583 | 0.605 |
| Cut-off | 3.99 | 3.83 | 3.31 | 3.37 | --- | 2.87 | --- | --- | --- | 2.71 | 2.67 | 2.18 | 1.59 | |
| SEN | 0.85 | 0.45 | 0.65 | 0.51 | --- | 0.52 | --- | --- | --- | 0.69 | 0.63 | 0.93 | 0.90 | |
| r | 0.511 * | 0.194 * | −0.511 * | 0.143 * | −0.068 | 0.032 | 0.063 | 0.040 | 0.049 | −0.074 | 0.001 | 0.253 * | ||
| TyG | AUC | 0.679 * | 0.908 * | 0.581 * | 0.503 | 0.684 * | 0.779 * | 0.520 | 0.507 | 0.431 | 0.621 * | 0.500 | 0.472 | 0.665 |
| Cut-off | 9.22 | 9.36 | 9.54 | 10.38 | 9.23 | 9.20 | --- | 9.29 | --- | 9.60 | 8.91 | --- | 8.05 | |
| SEN | 0.73 | 0.90 | 0.41 | 0.13 | 0.53 | 0.66 | --- | 0.49 | --- | 0.40 | 0.67 | --- | 0.83 | |
| R | 0.215 * | 0.743 * | −0.085 | 0.043 | 0.208 * | 0.377 * | 0.005 | 0.052 | 0.064 | −0.065 | 0.106* | 0.347 * | ||
| (Cut-Off) | Cardiometabolic Risk Factors | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRI-I (4.99) | CRI-II (2.47) | AC (3.99) | AIP (0.71) | TyG (9.22) | ||||||||||||||||
| Mixed-HC Population | No Risk | Risky | No Risk | Risky | No Risk | Risky | No Risk | Risky | No Risk | Risky | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| HC (Total) | 46 | 47.4 | 51 | 52.6 | 42 | 46.7 | 48 | 53.3 | 44 | 45.8 | 52 | 54.2 | 52 | 52.0 | 48 | 48.0 | 43 | 46.7 | 49 | 53.3 |
| HC/HT: † F. T4 | 33 | 47.8 | 36 | 52.2 | 30 | 44.1 | 38 | 55.9 | 32 | 47.1 | 36 | 52.9 | 36 | 52.2 | 33 | 47.8 | 29 | 46.0 | 34 | 54.0 |
| HC/DM: † A1c | 35 | 45.4 | 42 | 54.6 | 34 | 45.9 | 40 | 54.1 | 34 | 44.7 | 42 | 55.3 | 41 | 51.9 | 38 | 48.1 | 36 | 48.0 | 39 | 52.0 |
| HC/HD: † Vit.D | 25 | 53.2 | 22 | 46.8 | 21 | 48.8 | 22 | 51.2 | 23 | 50.0 | 23 | 50.0 | 28 | 57.1 | 21 | 42.9 | 27 | 57.4 | 20 | 42.6 |
| HC/HTN: † BP | 11 | 40.7 | 16 | 59.3 | 8 | 29.6 | 19 | 70.4 | 12 | 42.9 | 16 | 57.1 | 11 | 40.7 | 16 | 59.3 | 7 | 30.4 | 16 | 69.6 |
| HC/anemic–Hb | 14 | 60.9 | 9 | 39.1 | 8 | 40.0 | 12 | 60.0 | 14 | 60.9 | 9 | 39.1 | 16 | 64.0 | 9 | 36.0 | 14 | 60.9 | 9 | 39.1 |
| Chi2, df, P | 5.49, 1, (p = 0.019 *) | 0.06, 1, (p = 0.803 NS) | 5.23, 1, (p = 0.022 *) | 4.35, 1, (p = 0.039 *) | 7.54, 1, (p = 0.006 *) | |||||||||||||||
| HC/Males | 12 | 36.4 | 21 | 63.6 | 16 | 51.6 | 15 | 48.4 | 10 | 31.2 | 22 | 68.8 | 13 | 39.4 | 20 | 60.6 | 14 | 43.7 | 18 | 56.3 |
| HC/Females | 29 | 64.4 | 16 | 35.6 | 19 | 47.5 | 21 | 52.5 | 29 | 64.4 | 16 | 35.6 | 34 | 70.8 | 14 | 29.2 | 25 | 61.0 | 16 | 39.0 |
| HC/Age: <40 Y. | 4 | 66.7 | 2 | 33.3 | 1 | 16.7 | 5 | 83.3 | 4 | 66.7 | 2 | 33.3 | 4 | 66.7 | 2 | 33.3 | 2 | 40.0 | 3 | 60.0 |
| HC/Age: ≥40 Y. | 9 | 36.0 | 16 | 64.0 | 9 | 36.0 | 16 | 64.0 | 9 | 36.0 | 16 | 64.0 | 9 | 36.0 | 16 | 64.0 | 6 | 30.0 | 14 | 70.0 |
| HC/Obese: ≥30 kg/m2 | 2 | 33.3 | 4 | 66.7 | 2 | 33.3 | 4 | 66.7 | 2 | 33.3 | 4 | 66.7 | 2 | 33.3 | 4 | 66.7 | 0 | 0.0 | 6 | 100 |
| HC/O.W: 25–29.9 kg/m2 | 3 | 21.4 | 11 | 78.6 | 5 | 35.7 | 9 | 64.3 | 3 | 21.4 | 11 | 78.6 | 3 | 21.4 | 11 | 78.6 | 4 | 28.6 | 10 | 71.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.E.; Alsamghan, A.; Momenah, M.A.; Alqhtani, H.A.; Aldawood, N.A.; Alshehri, M.A.; Ali Alshehri, A.M.; Alhag, S.K.; Mosaad, Y.O.; Ahmed, H. Metabolic Syndrome and Cardiometabolic Risk Factors in the Mixed Hypercholesterolemic Populations with Respect to Gender, Age, and Obesity in Asir, Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 14985. https://doi.org/10.3390/ijerph192214985
Ahmed AE, Alsamghan A, Momenah MA, Alqhtani HA, Aldawood NA, Alshehri MA, Ali Alshehri AM, Alhag SK, Mosaad YO, Ahmed H. Metabolic Syndrome and Cardiometabolic Risk Factors in the Mixed Hypercholesterolemic Populations with Respect to Gender, Age, and Obesity in Asir, Saudi Arabia. International Journal of Environmental Research and Public Health. 2022; 19(22):14985. https://doi.org/10.3390/ijerph192214985
Chicago/Turabian StyleAhmed, Ahmed Ezzat, Awad Alsamghan, Maha Abdullah Momenah, Haifa Ali Alqhtani, Nouf Arkan Aldawood, Mohammed A. Alshehri, Abdulaziz Mohammad Ali Alshehri, Sadeq K. Alhag, Yasser O. Mosaad, and Hassan Ahmed. 2022. "Metabolic Syndrome and Cardiometabolic Risk Factors in the Mixed Hypercholesterolemic Populations with Respect to Gender, Age, and Obesity in Asir, Saudi Arabia" International Journal of Environmental Research and Public Health 19, no. 22: 14985. https://doi.org/10.3390/ijerph192214985