Dimeric (Poly)Hydroxynaphthazarins, Metabolites of Echinoderms and Lichens: The History of the Synthesis and Structure Elucidation
Abstract
:1. Introduction
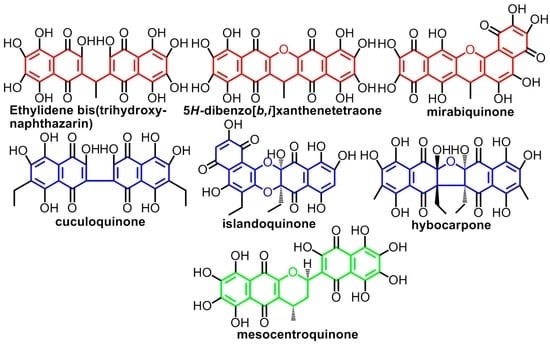
2. Types of Dimeric (Poly)Hydroxynaphthazarins
2.1. Aldol Condenation Compounds
2.2. Oxidative Coupling Compounds
2.3. Diene Condenstion Compounds
3. The Tautomerism of Hydroxynaphthazarins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thomson, R.H. Naturally Occurring Quinones, 2nd ed.; Academic Press: London, UK; New York, NY, USA, 1971. [Google Scholar]
- Thomson, R.H. Naturally Occurring Quinones, 3rd ed.; Chapman & Hall: London, UK, 1987. [Google Scholar]
- Thomson, R.H. Naturally Occurring Quinones, 4th ed.; Blackie Academic and Professional: London, UK; New York, NY, USA, 1997. [Google Scholar]
- Trisuwan, K.; Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Anthraquinone, Cyclopentanone, and naphthoquinone derivatives from the sea fan-derived fungi Fusarium spp. PSU-F14 and PSU-F135. J. Nat. Prod. 2010, 73, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Induli, M.; Cheloti, M.; Wasuna, A.; Wekesa, I.; Wanjohi, J.M.; Byamukama, R.; Heydenrich, M.; Makayoto, M.; Yenesew, A. Naphthoquinones from the roots of Aloe secundiflora. Phytochem. Lett. 2012, 5, 506–509. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Fonkeng, L.S.; Kopa, T.K.; Tala, M.F.; Wabo, H.K.; Tume, C.B.; Tane, P.; Kuiate, J.-R. Antibacterial activity of ethanolic extract and compounds from fruits of Tectona grandis (Verbenaceae). BMC Complement. Alternat. Med. 2015, 15, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, K.D.; Ellwood, N.; Kumar, R.; Yang, X.; Healy, P.C.; Choomuenwai, V.; Quinn, R.J.; Elliot, A.G.; Huang, J.X.; Chitty, J.L.; et al. Antibacterial and antifungal screening of natural products sourced from Australian fungi and characterization of pestalactams D-F. Phytochemistry 2016, 124, 79–85. [Google Scholar] [CrossRef]
- Silva, A.S.; Amorim, M.S.; Fonseca, M.M.; Salvador, M.J.; de Sá, E.L.; Stefanello, M.É.A. A New Cytotoxic Naphthoquinone and Other Chemical Constituents of Sinningia reitzii. J. Braz. Chem. Soc. 2019, 30, 2060–2065. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, J.; Sun, Z.; Liu, Y.; Sun, Z.; Zhu, N.; Xu, X.; Yang, J.; Ma, G. New naphtoquinones derivatives from the edible bulbs of Eleutherine americana and their protective effect on the injury of human umbilical vein endothelial cells. Fitoterapia 2019, 132, 46–52. [Google Scholar] [CrossRef]
- Lacret, R.; Varela, R.M.; Molinillo, J.M.G.; Nogueiras, C.; Macías, F.A. Anthratectone and naphthotectone, two quinones from bioactive extracts of Tectona grandis. J. Chem. Ecol. 2011, 37, 1341–1348. [Google Scholar] [CrossRef]
- Rauf, A.; Uddin, G.; Siddiqui, B.S.; Molnár, J.; Csonka, Á.; Ahmad, B.; Szabó, D.; Farooq, U.; Khan, A. A rare class of new dimeric naphthoquinones from Diospyros lotus have multidrug reversal and antiproliferative effects. Front. Pharmacol. 2015, 6, 293. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Hadda, T.B.; Patel, S.; Uddin, G.; Bawazeer, S.; Abu-Izneid, T.; Ahmad, B. Identification, structure elucidation, and antioxidant potential of a new compound from Diospyros lotus. Chem. Nat. Comp. 2017, 53, 849–851. [Google Scholar] [CrossRef]
- Iwata, D.; Ishibashi, M.; Yamamoto, Y. Cribrarione B, a New Naphthoquinone Pigment from the Myxomycete Cribraria cancellata. J. Nat. Prod. 2003, 66, 1611–1612. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, First Aminated Hydroxynaphthazarins from the Sea Urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef]
- Yakubovskaya, A.Y.; Pokhilo, N.D.; Mischenko, N.P.; Anufriev, V.P. Spinazarin and Ethylspinazarin, Pigments of the Sea Urchin Scaphechinus mirabilis. Russ. Chem. Bull. Int. Ed. 2007, 56, 819–822. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Qin, L.; Zhu, B.W.; Wang, X.D.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Wu, H.T.; Sun, L.M.; et al. Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin Strongylocentrotus nudus. Food Chem. 2011, 129, 1591–1597. [Google Scholar] [CrossRef]
- Powell, C.; Hughes, A.D.; Kelly, M.S.; Conner, S.; McDougall, G.J. Extraction and identification of antioxidant polyhydroxynaphthoquinone pigments from the sea urchin, Psammechinus miliaris. LWT-Food Sci. Technol. 2014, 59, 455–460. [Google Scholar] [CrossRef]
- Hou, Y.; Vasileva, E.A.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A.; Mishchenko, N.P. Naphthoquinones of the spinochrome class: Occurrence, isolation, biosynthesis and biomedical applications. RSC Adv. 2018, 8, 32637–32650. [Google Scholar] [CrossRef]
- Ageenko, N.V.; Kiselev, K.V.; Odintsova, N.A. Quinoid Pigments of Sea Urchins Scaphechinus mirabilis and Strongylocentrotus intermedius: Biological Activity and Potential Applications. Mar. Drugs 2022, 20, 611. [Google Scholar] [CrossRef]
- Mathieson, J.W.; Thomson, R.H. Naturally occurrinq naphthoquinones. Part XVIII. New spinochromes from Diadema antillarum, Spatangus purpureus, and Temnopleurus torenmaticus. J. Chem. Soc. (C) 1971, 153–160. [Google Scholar] [CrossRef]
- Kol’tsova, E.A.; Denisenko, V.A.; Maksimov, O.B. Quinoid pigments of echinoderms. V. Pigments of the sea urchin Strongylocentrotus dröebachiensis. Chem. Nat. Compd. 1978, 14, 371–374. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Tran, V.T.T.; Vo, H.M.N.; Bui, L.M.; Denisenko, V.A.; Fedoreyev, S.A. Quinoid Pigments from the Sea Urchin Astropyga radiate. Chem. Nat. Compd. 2017, 53, 356–358. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A. Mirabiquinone, a new unsymmetrical binaphthoquinone from the sea urchin Scaphechinus mirabilis. Tetrahedron Lett. 2014, 55, 5967–5969. [Google Scholar] [CrossRef]
- Pelageev, D.N.; Panchenko, M.N.; Pokhilo, N.D.; Denisenko, V.A.; Anufriev, V.P. Synthesis of lomazarin and norlomazarin, pigments from Lomandra hastilis. Chem. Nat. Comp. 2008, 44, 719–723. [Google Scholar] [CrossRef]
- Pelageev, D.N.; Panchenko, M.N.; Pokhilo, N.D.; Anufriev, V.P. Synthesis of 2,2′-(ethane-1,1-diyl)bis(3,5,6,7,8-pentahydroxynaphthoquinone)—A methobolite of sea urchins Spatangus purpureus, Strongylocentrotus intermedius and S. droebachiensis. Russ. Chem. Bull. Int. Ed. 2010, 59, 1472–1476. [Google Scholar] [CrossRef]
- Pelageev, D.N.; Anufriev, V.P. Synthesis of mirabiquinone A: A biquinone from the sea urchin Scaphechinus mirabilis and related compounds. Synthesis 2016, 48, 761–764. [Google Scholar]
- Krivoschekova, O.E.; Maximov, O.B.; Stepanenko, L.S.; Mishchenko, N.P. Quinones of the lichen Cetraria cucullata. Phytochemistry 1982, 21, 193–196. [Google Scholar] [CrossRef]
- Stepanenko, L.S.; Krivoshchekova, O.E.; Dmitrenok, P.S.; Maximov, O.B. Quinones of Cetraria islandica. Phytochemistry 1997, 46, 565–568. [Google Scholar] [CrossRef]
- Ernst-Russell, M.A.; Elix, J.A.; Chai, C.L.L.; Willis, A.C.; Hamada, N.; Nash, T.H.I. Hybocarpone, a novel cytotoxic naphthazarin derivative from mycobiont cultures of the lichen Lecanora hybocarpa. Tetrahedron Lett. 1999, 40, 6321–6324. [Google Scholar] [CrossRef]
- Glazunov, V.P.; Tchizhova, A.Y.; Shuvalova, M.I.; Anufriev, V.P. The chemistry of naphthazarin derivatives. 7. Determination of structure of substituted 2,6(7)-dihydroxynaphthazarins by UV- and IR-spectroscopy. Russ. Chem. Bull. Int. Ed. 2001, 50, 88–94. [Google Scholar] [CrossRef]
- Pokhilo, N.D.; Dragan, S.V.; Anufriev, V.P. Revision of the structure of cuculoquinone to 3,3′-bis(6-ethyl-2,5,7,8-tetrahydroxy-1,4-naphthoquinone) and confirmation of the proposed structure by synthesis. Tetrahedron Lett. 2011, 52, 3651–3653. [Google Scholar] [CrossRef]
- Bentley, R.; Campbell, I.M. Biological Reactions of Quinones. In The Chemistry of the Quinonoid Compounds; Patai, S., Ed.; Wiley-Interscience: London, UK, 1974. [Google Scholar]
- Torssell, K.B.G. Natural Product Chemistry; J. Wiley and Sons Limited: Hoboken, NJ, USA, 1983. [Google Scholar]
- Yamamoto, Y.; Matsubara, H.; Kinoshita, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Ahmadjiam, V.; Kurokawa, T.; Yoshimura, I. Naphthazarin derivayives from cultures of the lichen Cladonia cristatella. Phytochemistry 1996, 43, 1239–1242. [Google Scholar] [CrossRef]
- Naoe, A.; Ishibashi, M.; Yamamoto, Y. Cribrarione A, a new antimicrobial naphthoquinone pigment from a myxomycete Cribraria purpurea. Tetrahedron 2003, 59, 3433–3435. [Google Scholar] [CrossRef]
- Bringmann, G.; Rüdenauer, S.; Irmer, A.; Bruhn, T.; Brun, R.; Heimberger, T.; Stühmer, T.; Bargou, R.; Chatterjee, M. Antitumoral and antileishmanial dioncoquinones and ancistroquinones from cell cultures of Triphyophyllum peltatum (Dioncophyllaceae) and Ancistrocladus abbreviatus (Ancistrocladaceae). Phytochemistry 2008, 69, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, E.; Kucherak, O.; Stodůlkova, E.; Tosner, Z.; Císarova, I.; Flieger, M.; Kolarik, M.; Baszczynski, O. NMR Structure elucidation of naphthoquinones from Quambalaria cyanescens. J. Nat. Prod. 2021, 84, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ettlinger, M.J. Hydroxynaphthoquinones. III. The structure of lapachol peroxide. J. Am. Chem. Soc. 1950, 72, 3472–3474. [Google Scholar] [CrossRef]
- da Silva, E.N., Jr.; Pinto, M.C.F.R.; Moura, K.C.G.; Simone, C.A.; Nascimento, C.J.; Andrade, C.K.Z.; Pinto, A.V. Hooker’s ‘lapachol peroxide’ revisited. Tetrahedron Lett. 2009, 50, 1575–1577. [Google Scholar] [CrossRef]
- Yakubovskaya, A.Y.; Kochergina, T.Y.; Denisenko, V.A.; Berdyshev, D.V.; Glazunov, V.P.; Anufriev, V.P. Synthesis and Study on Oxidative Coupling Products of 2-Hydroxy-1,4-naphthoquinones. Russ. Chem. Bull. Int. Ed. 2006, 55, 301–305. [Google Scholar] [CrossRef]
- Tchizhova, A.Y.; Anufriev, V.P.; Glazunov, V.P.; Denisenko, V.A. The chemistry of naphthazarin derivatives. 6. Hydration of 2-oxo-2,3-dihydro-1,4-naphthoquinone derivatives in organic solvents. Russ. Chem. Bull. Int. Ed. 2000, 49, 466–471. [Google Scholar] [CrossRef]
- Tchizhova, A.Y.; Kochergina, T.Y.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. The Chemistry of Naphthazarin Derivatives 3. Synthesis of Dideoxy Analog of Islandoquinone. Russ. Chem. Bull. Int. Ed. 1999, 48, 938–943. [Google Scholar] [CrossRef]
- Tchizhova, A.Y.; Anufriev, V.P.; Glazunov, V.P.; Denisenko, V.A.; Moiseenko, O.P. Selective chlorination of hydroxynaphthazarins with dichlorine monoxide. Remarkable stability of some geminal diols derived from 2,3-dihydro-2-oxonaphthazarin. Synth. Commun. 1999, 29, 3971–3980. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian G03W, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Borisova, K.L.; Pelageev, D.N.; Kochergina, T.Y.; Pokhilo, N.D.; Pushilin, M.A.; Denisenko, V.A.; Berdyshev, D.V.; Anufriev, V.P. Concerning the structure of islandoquinone isolated from the lichen Cetraria islandica. Nat. Prod. Commun. 2014, 9, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, K.C.; Gray, D.L.F. Total synthesis of hybocarpone. Angew. Chem. Int. Ed. 2001, 40, 761–763. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Gray, D.L.F. Total synthesis of hybocarpone and analogues there of. A facile dimerization of naphthazarins to pentacyclic systems. J. Am. Chem. Soc. 2004, 126, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Hale, C.R.H.; Nilewskia, C.; Ioannidoua, H.A. Constructing molecular complexity and diversity: Total synthesis of natural products of biological and medicinal importance. Chem. Soc. Rev. 2012, 41, 5185–5238. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.L.L.; Elix, J.A.; Moore, F.K.E. An expedient and efficient synthetic route to some naturally occurring polyfunctional naphthazarins. Tetrahedron Lett. 2001, 42, 8915–8917. [Google Scholar] [CrossRef]
- Chai, C.L.L.; Elix, J.A.; Moore, F.K.E. Concise formal total synthesis of hybocarpone and related naturally occurring naphthazarins. J. Org. Chem. 2006, 71, 922–1001. [Google Scholar] [CrossRef]
- Wu, S.K.-L.; Cohen, E.P.M.T.; Huang, Y.; Pettus, T.R.R. First total synthesis of malvone a and formal syntheses of boryquinone and hybocarpone using a concise strategy for construction of unsymmetrical naphthoquinones. Synlett 2009, 8, 1273–1276. [Google Scholar]
- Chen, W.; Guo, R.; Yang, Z.; Gong, J. Formal total synthesis of hybocarpone enabled by visible-light-promoted benzannulation. J. Org. Chem. 2018, 83, 15524–15532. [Google Scholar] [CrossRef]
- Dragan, S.V.; Pushilin, M.A.; Glazunov, V.P.; Denisenko, V.A.; Anufriev, V.P. Total synthesis of hybocarpone, a cytotoxic naphthazarin derivative from the lichen Lecanora hybocarpa, and related compounds. Nat. Prod. Commun. 2014, 9, 1765–1768. [Google Scholar] [CrossRef]
- Glazunov, V.P.; Tchizhova, A.Y.; Shestak, O.P.; Sopelnyak, G.I.; Anufriev, V.P. The chemistry of naphthazarin derivatives. 8. Determination of structure of substituted 2-hydroxy-6(7)-methoxynaphthazarins, and 7(8)-hydroxypyranonaphthazarins by IR-spectroscopy. Russ. Chem. Bull. Int. Ed. 2001, 50, 95–100. [Google Scholar] [CrossRef]
- Pelageev, D.N.; Dragan, S.V.; Pushilin, M.A.; Denisenko, V.A.; Berdyshev, D.V.; Glazunov, V.P.; Anufriev, V.P. Synthesis and study on oxidative coupling products of 3-alkyl-2-hydroxynaphthazarins. Russ. Chem. Bull. Int. Ed. 2012, 61, 2102–2108. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623. [Google Scholar] [CrossRef]
- Dragan, S.V.; Borisova, K.L.; Pelageev, D.N.; Anufriev, V.P. Concerning the stereoselectivity of the oxidative dimerization of 3-alkyl-2-hydroxy-1,4-naphthoquinones in the synthesis of hybocarpone. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- de Silva, E.N., Jr.; Cavalcanti, B.C.; Guimarães, T.T.; Pinto, M.C.F.R.; Cabral, I.O.; Pessoa, C.; Costa-Lotufo, L.V.; de Moraes, M.O.; de Andrade, C.K.Z.; dos Santos, M.R.; et al. Synthesis and evaluation of quinonoid compounds against tumor cell lines. V. Eur. J. Med. Chem. 2011, 46, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Borisova, K.L.; Pelageev, D.N.; Melman, G.I.; Mashnev, B.P.; Anufriev, V.P. Synthesis of (+/–)-mesocentroquinone, a biquinone of a novel structural class and metabolite of sea urchins Mesocentrotus nudus and Strongylocentrotus intermedius, and related compounds. Chem. Nat. Compd. 2022, 58, 1006–1010. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Tran, V.T.T.; Vo, H.M.N.; Fedoreyev, S.A. Spinochrome identification and quantification in pacific sea urchin shells, coelomic fluid and eggs using HPLC-DAD-MS. Mar. Drugs 2021, 19, 21. [Google Scholar] [CrossRef]
- Moore, R.E.; Scheuer, P.J. Nuclear magnetic resonance spectra of substituted naphthoquinones. Influence of substituents on tautomerism, anisotropy, and stereochemistry in the naphthazarin system. J. Org. Chem. 1966, 31, 3272–3283. [Google Scholar] [CrossRef] [PubMed]
- Glazunov, V.P.; Tchizchova, A.Y.; Pokhilo, N.D.; Anufriev, V.P.; Elyakov, G.B. First Direct Observation of Tautomerism of Monohydroxynaphthazarins by IR-Spectroscopy. Tetrahedron 2002, 58, 1751–1757. [Google Scholar] [CrossRef]
- Glazunov, V.P.; Yakubovskaya, A.Y.; Pokhilo, N.D.; Bochinskaya, N.V.; Anufriev, V.P. Chemistry of naphthazarin derivatives 9. Direct observation of prototropic tautomerism of (poly)hydroxynaphthazarins by IR-spectroscopy. Russ. Chem. Bull. Int. Ed. 2003, 52, 198–207. [Google Scholar] [CrossRef]
- Mariam, Y.H.; Musin, R.N. A B3LYP study of intramolecular hydrogen bonding and proton transfer in naphthazarin: A model system for daunomycin/adriamycin. J. Mol. Struct. (Theochem) 2001, 549, 123–136. [Google Scholar] [CrossRef]
- Glazunov, V.P.; Pokhilo, N.D.; Bochinskaya, N.V.; Yakubovskaya, A.Y.; Anufriev, V.P. Chemistry of naphthazarin derivatives. 10. First direct observation of prototropic tautomerism of 1′-hydroxyalkylnaphthazarins by IR spectroscopy. Russ. Chem. Bull. Int. Ed. 2003, 52, 1629–1632. [Google Scholar] [CrossRef]
- Shikov, A.N.; Ossipov, V.I.; Martiskainen, O.; Pozharitskaya, O.N.; Ivanova, S.A.; Makarov, V.G. The offline combination of thin-layer chromatography and high-performance liquid chromatography with diode array detection and micrOTOF-Q mass spectrometry for the separation and identification of spinochromes from sea urchin (Strongylocentrotus droebachiensis) shells. J. Chromatogr. A 2011, 1218, 9111–9114. [Google Scholar]
- Kuroda, C.; Ohshima, H. The pigments from the sea urchins and the synthesis of the related compounds. Proc. Imp. Acad. 1940, 16, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Lederer, E. Sur les pigments naphthoquinoniques des epines et du test des oursins Paracentrotus lividus et Arbacia pustulosa. Biochim. Biophis. Acta 1952, 9, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, C.; Okajima, M. Studies on the derivatives of naphthoquinones. XII. The pigments from the sea urchins. Proc. Jpn. Acad. 1954, 30, 982–986. [Google Scholar] [CrossRef] [Green Version]



























| Compounds | δH(CH2(15)) | δH(CH3 (16)) | δH(Ha(17)) | δH(Hb(17)) | δH(CH3(18)) | δC(C-6a) | δC(C-7a) | δC(C-13a) | δC(C-14a) |
|---|---|---|---|---|---|---|---|---|---|
| islandoquinone | 2.96 | 1.37 | 1.79 | 2.36 | 1.05 | 146.0 | 83.0 | 93.0 | 145.0 |
| 23a | 2.95 | 1.35 | 1.79 | 2.37 | 1.05 | 147.2 | 83.7 | 92.3 | 144.2 |
| 23b | 2.92 | 1.37 | 1.75 | 2.36 | 1.04 | 146.1 | 82.6 | 92.4 | 136.2 |
| 23c | 2.55 | 0.97 | 1.69 | 2.34 | 1.17 | 144.2 | 92.8 | 83.1 | 138.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelageev, D.N.; Borisova, K.L.; Anufriev, V.P. Dimeric (Poly)Hydroxynaphthazarins, Metabolites of Echinoderms and Lichens: The History of the Synthesis and Structure Elucidation. Mar. Drugs 2023, 21, 407. https://doi.org/10.3390/md21070407
Pelageev DN, Borisova KL, Anufriev VP. Dimeric (Poly)Hydroxynaphthazarins, Metabolites of Echinoderms and Lichens: The History of the Synthesis and Structure Elucidation. Marine Drugs. 2023; 21(7):407. https://doi.org/10.3390/md21070407
Chicago/Turabian StylePelageev, Dmitry N., Ksenia L. Borisova, and Victor Ph. Anufriev. 2023. "Dimeric (Poly)Hydroxynaphthazarins, Metabolites of Echinoderms and Lichens: The History of the Synthesis and Structure Elucidation" Marine Drugs 21, no. 7: 407. https://doi.org/10.3390/md21070407