Fine-Dust-Induced Skin Inflammation: Low-Molecular-Weight Fucoidan Protects Keratinocytes and Underlying Fibroblasts in an Integrated Culture Model
Abstract
:1. Introduction
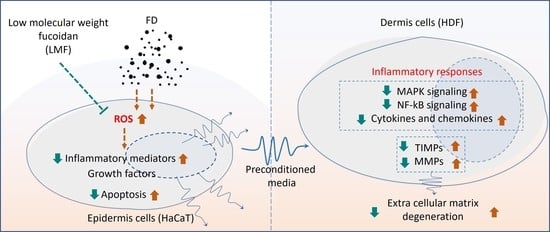
2. Results
2.1. LMF Effectively Increased Cell Viability and Abated Oxidative Stress in the Integrated-Culture Model
2.2. LMF Suppressed Apoptosis in FD-Stimulated HaCaT Keratinocytes and HDFs in the Integrated-Culture Model
2.3. LMF Effectively Downregulated the NF-κB/MAPK Signaling in FD-Stimulated HaCaT Keratinocytes and HDFs in the Integrated-Culture Model
2.4. Preconditioned Media Downregulated Inflammatory Mediators in HDFs in the Integrated-Culture Model
2.5. Preconditioned Media Downregulated MMPs and TIMPs, and Suppressed Collagenase and Elastase Activity in HDFs in the Integrated-Culture Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Purification Method of LMF from S. confusum
4.3. HaCaT Keratinocyte-HDF Integrated Cell Culture, FD Stimulation, and LMF Treatment
4.4. Investigation of Cell Viability
4.5. Analysis of Intracellular ROS Production
4.6. Investigation of Apoptosis
4.7. Western Blot Analysis
4.8. RNA Extraction and RT-PCR Analysis
4.9. ELISA Analysis
4.10. Investigation of Intracellular Collagenase and Elastase Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jayawardena, T.U.; Asanka Sanjeewa, K.; Shanura Fernando, I.; Ryu, B.M.; Kang, M.-C.; Jee, Y.; Lee, W.W.; Jeon, Y.-J. Sargassum horneri (Turner) C. Agardh ethanol extract inhibits the fine dust inflammation response via activating Nrf2/HO-1 signaling in RAW 264.7 cells. BMC Complement. Altern. Med. 2018, 18, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Kim, H.-S.; Fernando, I.P.S.; Ahn, G. (−)-Loliolide isolated from sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W. Air pollution and skin disorders. Int. J. Women’s Dermatol. 2021, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Ho, C.-H.; Kim, J.-H.; Kim, J. Quiescence of Asian dust events in South Korea and Japan during 2012 spring: Dust outbreaks and transports. Atmos. Environ. 2015, 114, 92–101. [Google Scholar] [CrossRef]
- Pozzi, R.; De Berardis, B.; Paoletti, L.; Guastadisegni, C. Inflammatory mediators induced by coarse (PM2.5–10) and fine (PM2.5) urban air particles in RAW 264.7 cells. Toxicology 2003, 183, 243–254. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Kim, H.-S.; Han, E.-J.; Kim, M.-J.; Seo, M.-J.; Ahn, G. Effects of (–)-Loliolide against Fine Dust Preconditioned Keratinocyte Media-Induced Dermal Fibroblast Inflammation. Antioxidants 2021, 10, 675. [Google Scholar] [CrossRef]
- Savitz, D.A.; Elston, B.; Bobb, J.F.; Clougherty, J.E.; Dominici, F.; Ito, K.; Johnson, S.; McAlexander, T.; Ross, Z.; Shmool, J.L.; et al. Ambient fine particulate matter, nitrogen dioxide, and hypertensive disorders of pregnancy in New York City. Epidemiology 2015, 26, 748. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Archer, C. Functions of the skin. In Rook’s Textbook of Dermatology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; Volume 1, pp. 1–11. [Google Scholar]
- Bangert, C.; Brunner, P.M.; Stingl, G. Immune functions of the skin. Clin. Dermatol. 2011, 29, 360–376. [Google Scholar] [CrossRef]
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery 2010, 28, 469–472. [Google Scholar]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.-J.; Lee, K.; Cheong, S.H.; Ahn, G. Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. Int. J. Biol. Macromol. 2021, 168, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.; Kim, H.-S.; Sanjeewa, K.; Oh, J.-Y.; Jeon, Y.-J.; Lee, W.W. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macrophages by diphlorethohydroxycarmalol isolated from a brown alga Ishige okamurae. Algae 2017, 32, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Jayasinghe, A.M.K.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Han, E.J.; Oh, G.-W.; Jung, W.-K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Han, E.-J.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Kim, E.-A.; Kim, J.; Jung, K.; Kim, K.-N.; Heo, S.-J.; Ahn, G. 3-Bromo-4, 5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-α/IFN-γ-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes. Mar. Drugs 2022, 20, 563. [Google Scholar] [CrossRef]
- Shin, D.-B.; Han, E.-H.; Park, S.-S. Cytoprotective effects of Phaeophyta extracts from the coast of Jeju island in HT-22 mouse neuronal cells. J. Korean Soc. Food Sci. Nutr. 2014, 43, 224–230. [Google Scholar] [CrossRef]
- Khan, M.N.; Choi, J.S.; Lee, M.C.; Kim, E.; Nam, T.J.; Fujii, H.; Hong, Y.K. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol. 2008, 29, 465–469. [Google Scholar]
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Wang, H.-M.; Chiu, C.-C.; Wu, P.-F.; Chen, C.-Y. Subamolide E from Cinnamomum subavenium induces sub-G1 cell-cycle arrest and caspase-dependent apoptosis and reduces the migration ability of human melanoma cells. J. Agric. Food Chem. 2011, 59, 8187–8192. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Qin, Y. Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Atef, M.; Ojagh, S.M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Priyan Shanura Fernando, I.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Herath, K.H.; Yang, H.-W.; Choi, C.S.; Jeon, Y.-J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.; Biller, P.; Ross, A.; Adams, J. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Xue, C.-H.; Li, Z.-J.; Cai, Y.-P.; Liu, H.-Y.; Qi, H.-T. Antioxidant and hepatoprotective activities of low molecular weight sulfated polysaccharide from Laminaria japonica. J. Appl. Phycol. 2004, 16, 111–115. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R. Human keratinocyte UVB-protective effects of a low molecular weight fucoidan from Sargassum horneri purified by step gradient ethanol precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Jing, R.; Guo, K.; Zhong, Y.; Wang, L.; Zhao, J.; Gao, B.; Ye, Z.; Chen, Y.; Li, X.; Xu, N. Protective effects of fucoidan purified from Undaria pinnatifida against UV-irradiated skin photoaging. Ann. Transl. Med. 2021, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Majid, M.; Haq, I.-u.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ren, D.; Song, Y.; Wu, L.; He, Y.; Peng, Y.; Zhou, H.; Liu, S.; Cong, H.; Zhang, Z. Gastric protective activities of fucoidan from brown alga Kjellmaniella crassifolia through the NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 149, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Che, N.; Ma, Y.; Xin, Y. Protective role of fucoidan in cerebral ischemia-reperfusion injury through inhibition of MAPK signaling pathway. Biomol. Ther. 2017, 25, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, I.; Sun, Z.; Ukachi, M.; Nagano, K.; McLeod, C.W.; Cox, A.G.; Nishikawa, M. Development and certification of the new NIES CRM 28: Urban aerosols for the determination of multielements. Anal. Bioanal. Chem. 2008, 391, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Wang, L.; Jeon, Y.-J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Kim, H.S.; Wang, L.; Lee, W.W.; Jeon, Y.J. Apoptotic and antiproliferative properties of 3β-hydroxy-∆5-steroidal congeners from a partially purified column fraction of Dendronephthya gigantea against HL-60 and MCF-7 cancer cells. J. Appl. Toxicol. 2018, 38, 527–536. [Google Scholar] [CrossRef]







| Target Gene | Primer Sequence (5′ to 3′ Direction) | |
|---|---|---|
| IL-1β | Forward | TGT CCT GCG TGT TGA AAG ATG A |
| Reverse | CAG GCA GTT GGG CAT TGG TG | |
| IL-6 | Forward | GAT GGC TGA AAA AGA TGG ATG C |
| Reverse | TGG TTG GGT CAG GGG TGG TT | |
| IL-8 | Forward | ACA CTG CGC CAA CAC AGA AAT TA |
| Reverse | CAG GCA GTT GGG CAT TGG TG | |
| IL-33 | Forward | GAT GAG ATG TCT CGG CTG CTT G |
| Reverse | AGC CGT TAC GGA TAT GGT GGT C | |
| TNF-α | Forward | GGC AGT CAG ATC ATC TTC TCG AA |
| Reverse | GAA GGC CTA AGG TCC ACT TGT GT | |
| MMP 1 | Forward | CTG AAG GTG ATG AAG CAG CC |
| Reverse | AGT CCA AGA GAA TGG CCG AG | |
| MMP 2 | Forward | GCG ACA AGA AGT ATG GCT TC |
| Reverse | TGC CAA GGT CAA TGT CAG GA | |
| MMP 3 | Forward | CTC ACA GAC CTG ACT CGG TT |
| Reverse | CAC GCC TGA AGG AAG AGA TG | |
| MMP 8 | Forward | ATG GAC CAA CAC CTC CGC AA |
| Reverse | GTC AAT TGC TTG GAC GCT GC | |
| MMP 9 | Forward | CGC AGA CAT CGT CAT CCA GT |
| Reverse | GGA TTG GCC TTG GAA GAT GA | |
| MMP 13 | Forward | CTA TGG TCC AGG AGA TGA AG |
| Reverse | AGA GTC TTG CCT GTA TCC TC | |
| TIMP 1 | Forward | TTC TGG CAT CCT GTT GTT GCT |
| Reverse | CCT GAT GAC GAG GTC GGA ATT | |
| TIMP 2 | Forward | TGG AAA CGA CAT TTA TGG CAA CCC |
| Reverse | CTC CAA CGT CCA GCG AGA CC | |
| GAPDH | Forward | CGT CTA GAA AAA CCT GCC AA |
| Reverse | TGA AGT CAA AGG AGA CCA CC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Cho, N.; Cho, S.H.; Yoo, H.M.; Fernando, I.P.S.; Ahn, G. Fine-Dust-Induced Skin Inflammation: Low-Molecular-Weight Fucoidan Protects Keratinocytes and Underlying Fibroblasts in an Integrated Culture Model. Mar. Drugs 2023, 21, 12. https://doi.org/10.3390/md21010012
Kirindage KGIS, Jayasinghe AMK, Cho N, Cho SH, Yoo HM, Fernando IPS, Ahn G. Fine-Dust-Induced Skin Inflammation: Low-Molecular-Weight Fucoidan Protects Keratinocytes and Underlying Fibroblasts in an Integrated Culture Model. Marine Drugs. 2023; 21(1):12. https://doi.org/10.3390/md21010012
Chicago/Turabian StyleKirindage, Kirinde Gedara Isuru Sandanuwan, Arachchige Maheshika Kumari Jayasinghe, Namki Cho, Seok Ho Cho, Hee Min Yoo, Ilekuttige Priyan Shanura Fernando, and Ginnae Ahn. 2023. "Fine-Dust-Induced Skin Inflammation: Low-Molecular-Weight Fucoidan Protects Keratinocytes and Underlying Fibroblasts in an Integrated Culture Model" Marine Drugs 21, no. 1: 12. https://doi.org/10.3390/md21010012