Alginates Combined with Natural Polymers as Valuable Drug Delivery Platforms
Abstract
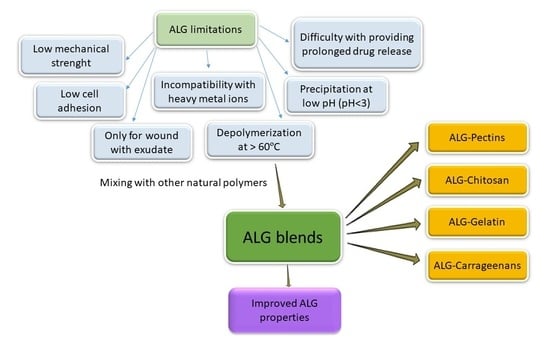
:1. Introduction
2. The Chemical Structure and Physical Properties of ALG
The Gelling Properties of ALG
3. Biological Properties of ALG
4. Pharmaceutical Applications of ALG
4.1. Microparticles
4.2. Nanoparticles
4.3. Tablet Technology
5. Biomedical Applications of ALG
5.1. Tissue Regeneration
5.2. Wound Care
6. Utilization of ALG Mixed with Selected Naturally Derived Polymers
6.1. ALG Blends with Pectins
6.2. ALG Blends with Chitosan
6.3. ALG Blends with Gelatin
6.4. ALG Blends with Carrageenans
| ALG Blends | Drug Form | Effect | Reference |
|---|---|---|---|
| ALG–PEC | Microcapsules Films Particles | Increased encapsulation efficiency Loss of spherical shape Improved drug retention Release prolongation Increased elongation at break Maintaining shape after rehydration Increased tensile strength Decreased gel strength Increased swelling Increased encapsulation efficiency Increased mucoadhesion Release prolongation | [176,177,178] [176,177,178] [176,178] [177,178] [182,184] [182] [184] [183] [183] [183,185,186] [185,186] [185,186] |
| ALG–CH | Dressings Bone defect filling Particles Films Inserts | Increased antibacterial effect Increased anti-inflammatory effect Reduced brittleness Increased Young modulus Release prolongation Increased folding resistance and tensile strength Increased bioadhesion strength and time Release prolongation Increased mucoadhesion Release prolongation | [195] [195,196] [197] [197] [198,199] [200] [200] [200] [201] [201] |
| ALG–GEL | Microspheres Injectable subcutaneous hydrogel Microcapsules Films Matrix tablets Dressing | Increased proliferation and differentiation Gelation time prolongation Increased swelling Increased degradability Improved cell adhesion, proliferation and survival Release reduction Mechanical properties improvement Increased degradability, cell adhesion Increased swelling | [210] [212] [212] [213] [214] [215] [217,220] [217] [220] |
| ALG–CAR | Particles Dressings Microcapsules Bio ink for 3D printing | Increased swelling Increased release Release prolongation Increased swelling Increased pore size Increased tensile strength and Young’s modulus Improvement in wound healing Protection against acidic pH Shape fidelity Increased shear modulus | [236] [236,242] [242] [237] [237] [238] [238] [239] [241] [241] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Kloareg, B.; Quatrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 259–315. [Google Scholar]
- Myklestad, S. Ion-exchange properties of brown algae I. Determination of rate mechanism for calciumhydrogen ion exchange for particles from Laminaria hyperborea and Laminaria digitata. J. Appl. Chem. 1968, 18, 30–36. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 1, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Repka, M.A.; Singh, A. Alginic acid. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; pp. 20–22. [Google Scholar]
- Cable, C.G. Sodium alginate. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; pp. 622–624. [Google Scholar]
- Gable, C.G. Calcium alginate. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; pp. 83–85. [Google Scholar]
- Shahand, S.A.; Thassu, D. Ammonium alginate. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; p. 41. [Google Scholar]
- Nause, R.G.; Reddy, R.D.; Soh, J.L.P. Propylene glycol alginate. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; pp. 594–595. [Google Scholar]
- Sanchez-Ballester, N.M.; Bataille, B.; Soulairol, I. Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship. Carbohydr. Polym. 2021, 270, 118399. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial alginate production: An overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium alginate as a pharmaceutical excipient: Novel applications of a well-known polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Vicente, A.A. Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 11, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Skjåk Bræk, G.; Smidsrød, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Draget, K.I.; Simensen, M.K.; Onsøyen, E.; Smidsrød, O. Gel strength of Ca-limited alginate gels made in situ. Hydrobiologia 1993, 260–261, 563–565. [Google Scholar] [CrossRef]
- Fu, S.; Thacker, A.; Sperger, D.M.; Boni, R.L.; Buckner, I.S.; Velankar, S.; Munson, E.J.; Block, L.H. Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS PharmSciTech 2011, 12, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Niekraszewicz, B.; Niekraszewicz, A. The structure of alginate, chitin and chitosan fibres. In Handbook of Textile Fibre Structure; Eichhorn, S.J., Hearle, J.W.S., Jaffe, M., Kikutani, T., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; Volume 2, pp. 266–304. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Mirghani, A.; Idkaidek, N.M.; Salem, M.S.; Najib, N.M. Formulation and release behavior of diclofenac sodium in Compritol 888 matrix beads encapsulated in alginate. Drug Dev. Ind. Pharm. 2000, 26, 791–795. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacrmolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-thaw induced gelation of alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Generally Recognized as Safe (Internet), U.S. Food and Drug Administration. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260857.htm (accessed on 2 November 2022).
- Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/inactive-ingredients-database-download (accessed on 2 November 2022).
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadsadeghi, A.; Farjadian, F.; Alipour, S. Sustained release of linezolid in ocular insert based on lipophilic modified structure of sodium alginate. Iran. J. Basic Med. Sci. 2021, 24, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A. Alginate particles as platform for drug delivery by the oral route: State-of-the-art. ISRN Pharm. 2014, 2014, 17. [Google Scholar] [CrossRef] [Green Version]
- Yavorska, N. Sodium alginate—A potential tool for weight management: Effect on subjective appetite, food intake, and glycemic and insulin regulation. JULS 2012, 6, 66–69. [Google Scholar]
- El Khoury, D.; Goff, H.D.; Berengut, S.; Kubant, R.; Anderson, G.H. Effect of sodium alginate addition to chocolate milk on glycemia, insulin, appetite and food intake in healthy adult men. Eur. J. Clin. Nutr. 2014, 68, 613–618. [Google Scholar] [CrossRef]
- El Khoury, D.; Goff, H.D.; Anderson, G.H. The role of alginates in regulation of food intake and glycemia: A gastroenterological perspective. Crit. Rev. Food Sci. Nutr. 2015, 55, 1406–1424. [Google Scholar] [CrossRef]
- Szekalska, M.; Wróblewska, M.; Sosnowska, K.; Winnicka, K. Influence of sodium alginate on hypoglycemic activity of metformin hydrochloride in the microspheres obtained by the spray drying. Int. J. Polym. Sci. 2016, 12, 8635408. [Google Scholar] [CrossRef] [Green Version]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef]
- Guo, L.; Xia, J.; Yu, S.; Yan, J.; He, F.; Zhang, M.; Fan, Q.; Yang, R.; Zhao, W. Natural edible materials made of protein-functionalized aerogel particles for postprandial hyperglycemia management. Int. J. Biol. Macromol. 2021, 167, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Idota, Y.; Kogure, Y.; Kato, T.; Ogawa, M.; Kobayashi, S.; Kakinuma, C.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; et al. Cholesterol-lowering effect of calcium alginate in rats. Biol. Pharm. Bull. 2016, 39, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Kim, S.-K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Lee, J.-B.; Takeshita, A.; Hayashi, K.; Hayashi, T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011, 86, 995–999. [Google Scholar] [CrossRef]
- Yan, G.L.; Guo, Y.M.; Yuan, J.M.; Liu, D.; Zhang, B.K. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult. Sci. 2011, 90, 1441–1448. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Khan, S.; Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsøyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E.; et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, M.F.; Powell, L.C.; Menzies, G.E.; Lewis, P.D.; Hawkins, K.; Wright, C.; Doull, I.; Walsh, T.R.; Onsøyen, E.; Dessen, A.; et al. A new class of safe oligosaccharide polymer therapy to modify the mucus barrier of chronic respiratory disease. Mol. Pharm. 2016, 13, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Complement. Integr. Med. 2020, 17, 20190203. [Google Scholar] [CrossRef]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed. Res. Int. 2015, 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-X.; Zhang, X.-S.; Guan, H.-S.; Wang, W. Potential anti-HPV and related cancer agents from marine resources: An overview. Mar. Drugs. 2014, 12, 2019–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral properties of alginate-based biomaterials: Promising antiviral agents against SARS-CoV-2. ACS Appl. Bio. Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Fu, G.; Wang, K.; Yang, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Advances in research on antiviral activities of sulfated polysaccharides from seaweeds. Pharmaceuticals 2022, 15, 581. [Google Scholar] [CrossRef] [PubMed]
- Heidarieh, M.; Soltani, M.; Tamimi, A.H.; Toluei, M.H. Comparative effect of raw fiber (Vitacel) and alginic acid (Ergosan) on growth performance, immunocompetent cell population and plasma lysozyme content of giant sturgeon (Huso huso). Turk. J. Fish. Aquat. 2011, 11, 445–450. [Google Scholar] [CrossRef]
- Bagni, M.; Romano, N.; Finoia, M.G.; Abelli, L.; Scapigliati, G.; Tiscar, P.G.; Sarti, M.; Marino, G. Short- and long-term effects of a dietary yeast beta-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 2005, 18, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prakash, C.; Chadha, N.K.; Gupta, S.K.; Jain, K.K.; Pandey, P.K. Effects of dietary alginic acid on growth and haemato-immunological responses of Cirrhinus mrigala (Hamilton, 1822) fingerlings. Turk. J. Fish. Aquat. Sc. 2019, 19, 373–382. [Google Scholar]
- Sarithakumari, C.H.; Renju, G.L.; Kurup, G.M. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268. [Google Scholar] [CrossRef]
- Sarithakumari, C.H.; Kurup, G.M. Alginic acid isolated from Sargassum wightii exhibits anti-inflammatory potential on type II collagen induced arthritis in experimental animals. Int. Immunopharmacol. 2013, 17, 1108–1115. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Jeon, Y.J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, S.A.; Moon, P.D.; Na, H.J.; Park, R.K.; Um, J.Y.; Kim, H.M.; Hong, S.H. Alginic acid has anti-anaphylactic effects and inhibits inflammatory cytokine expression via suppression of nuclear factor-κB activation. Clin. Exp. Allergy. 2006, 36, 785–794. [Google Scholar] [CrossRef]
- Uno, T.; Hattori, M.; Yoshida, T. Oral administration of alginic acid oligosaccharide suppresses IgE production and inhibits the induction of oral tolerance. Biosci. Biotechnol. Biochem. 2006, 70, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Ji, W.; Du, J.-R.; Yu, D.K.; He, Y.; Yu, C.X.; Li, D.S.; Zhao, C.Y.; Qiao, K.Y. Preventive effects of low molecular mass potassium alginate extracted from brown algae on DOCA salt-induced hypertension in rats. Biomed. Pharmacother. 2010, 64, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. The European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- The United States Pharmacopeia. “The United States Pharmacopeial Convention”, USP 44-NF 39; The United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterization. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Kheiri, M.T.; Abnous, K.; Eskandari, M.; Tafaghodi, M. Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: Dry powder formulation for nasal immunization in rabbits. Microb. Pathog. 2018, 115, 74–85. [Google Scholar] [CrossRef]
- Wong, T.W.; Dhanawat, M.; Rathbone, M.J. Vaginal drug delivery: Strategies and concerns in polymeric nanoparticle development. Expert. Opin. Drug. Deliv. 2014, 11, 1419–1434. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Narayan, P.; Liu, G.; Panyam, J. Polymer-surfactant nanoparticles for improving oral bioavailability of doxorubicin. J. Pharm. Investig. 2017, 47, 65–73. [Google Scholar] [CrossRef]
- Bowey, K.; Neufeld, R.J. Systemic and mucosal delivery of drugs within polymeric microparticles produced by spray drying. BioDrugs 2010, 24, 359–377. [Google Scholar] [CrossRef]
- Lopes, M.; Abrahim, B.; Veiga, F.; Seiça, R.; Cabral, M.R.; Arnaud, P.; Andrade, J.C.; Ribeiro, A.J. Preparation methods and applications behind alginate-based particles. Expert. Opin. Drug Deliv. 2017, 14, 769–782. [Google Scholar] [CrossRef]
- Ranjan, S.; Fontana, F.; Ullah, H.; Hirvonen, J.; Santos, H.A. Microparticles to enhance delivery of drugs and growth factors into wound sites. Ther. Deliv. 2016, 7, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, D.T.; Brannon-Peppas, L. Microparticle Drug Delivery Systems. In Drug Delivery Systems in Cancer Therapy. Cancer Drug Discovery and Development; Brown, D.M., Ed.; Humana Press: Totowa, NJ, USA, 2004; pp. 117–135. [Google Scholar] [CrossRef]
- Patel, R.P.; Baria, A.H.; Pandya, N.B. Stomach-specific drug delivery of famotidine using floating alginate beads. Int. J. PharmTech Res. 2009, 1, 288–291. [Google Scholar]
- Patel, N.; Lalwani, D.; Gollmer, S.; Injeti, E.; Sari, Y.; Nesamony, J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog. Biomater. 2016, 5, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.N.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- Springer, M.L.; Hortelano, G.; Bouley, D.M.; Wong, J.; Kraft, P.E.; Blau, H.M. Induction of angiogenesis by implantation of encapsulated primary myoblasts expressing vascular endothelial growth factor. J. Gene. Med. 2000, 2, 279–288. [Google Scholar] [CrossRef]
- Tan, H.; Huang, D.; Lao, L.; Gao, C. RGD modified PLGA/gelatin microspheres as microcarriers for chondrocyte delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 228–238. [Google Scholar] [CrossRef]
- Patil, S.B.; Sawant, K.K. Mucoadhesive microspheres: A promising tool in drug delivery. Curr. Drug Deliv. 2008, 5, 312–318. [Google Scholar] [CrossRef]
- Basmanav, B.F.; Kose, G.T.; Hasirci, V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials 2008, 29, 4195–4204. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Neufeld, R.J.; Arnaud, P.; Chaumeil, J.C. Microencapsulation of lipophilic drugs in chitosan-coated alginate microspheres. Int. J. Pharm. 1999, 187, 115–123. [Google Scholar] [CrossRef]
- Zhang, C.; Grossier, R.; Candoni, N.; Veesler, S. Preparation of alginate hydrogel microparticles by gelation introducing cross-linkers using droplet-based microfluidics: A review of methods. Biomater. Res. 2021, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Hu, J.J. Sub-100-micron calcium-alginate microspheres: Preparation by nitrogen flow focusing, dependence of spherical shape on gas streams and a drug carrier using acetaminophen as a model drug. Carbohydr Polym. 2021, 269, 118262. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Correia, C.; Malpique, R.; Brito, C.; Jensen, J.; Bjorquist, P.; Carrondo, M.J.; Alves, P.M. Microencapsulation technology: A powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS ONE 2011, 6, e23212. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Wang, P.; Guo, Y.; Xiang, L.; Yang, Y.; Qu, Y.; Gong, P.; Deng, L. Angiogenic and osteogenic potential of platelet-rich plasma and adipose-derived stem cell laden alginate microspheres. Biomaterials 2012, 33, 8802–8811. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Du, K.T.; Fang, Q.; Gu, Y.; Mihardja, S.S.; Sievers, R.E.; Wu, J.C.; Lee, R.J. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 2010, 31, 7012–7020. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Martín, M.J.; Calpena, A.C.; Fernández, F.; Mallandrich, M.; Gálvez, P.; Clares, B. Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohydr. Polym. 2015, 117, 140–149. [Google Scholar] [CrossRef]
- Benavides, S.; Cortes, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef]
- Faidi, A.; Lassoued, M.A.; Becheikh, M.E.H.; Touati, M.; Stumbé, J.F.; Farhat, F. Application of sodium alginate extracted from a Tunisian brown algae Padina pavonica for essential oil encapsulation: Microspheres preparation, characterization and in vitro release study. Int. J. Biol. Macromol. 2019, 136, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz, M.; Lopez, A.; Franco, E.; Garcia-Garcia, D.; Fenollar, D.; Balart, R. Development and characterization of bioactive alginate microcapsules with cedarwood essential oil. Flavour Fragran. J. 2017, 32, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Hussein, N.; Omer, H.; Ismael, A.; Albed Alhnan, M.; Elhissi, A.; Ahmed, W. Spray-dried alginate microparticles for potential intranasal delivery of ropinirole hydrochloride: Development, characterization and histopathological evaluation. Pharm. Dev. Technol. 2020, 25, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, A.B.; Silvestre, A.L.P.; Sposito, L.; de Souza, M.P.C.; Sábio, R.M.; Araújo, V.H.S.; Cury, B.S.F.; Chorilli, M. The role of polysaccharides from natural resources to design oral insulin micro- and nanoparticles intended for the treatment of Diabetes mellitus: A review. Carbohydr. Polym. 2021, 256, 117504. [Google Scholar] [CrossRef]
- Reis, C.P.; Ribeiro, A.J.; Neufeld, R.J.; Veiga, F. Alginate microparticles as novel carrier for oral insulin delivery. Biotechnol. Bioeng. 2007, 96, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Builders, P.F.; Kunle, O.O.; Okpaku, L.C.; Builders, M.I.; Attama, A.A.; Adikwu, M.U. Preparation and evaluation of mucinated sodium alginate microparticles for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2008, 70, 777–783. [Google Scholar] [CrossRef]
- Bowey, K.; Swift, B.E.; Flynn, L.E.; Neufeld, R.J. Characterization of biologically active insulin-loaded alginate microparticles prepared by spray drying. Drug. Dev. Ind. Pharm. 2013, 39, 457–465. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Papain entrapment in alginate beads for stability improvement and site-specific delivery: Physicochemical characterization and factorial optimization using neural network modeling. AAPS PharmSciTech 2005, 6, E209–E222. [Google Scholar] [CrossRef] [Green Version]
- Simi, C.K.; Emilia Abraham, T. Encapsulation of crosslinked subtilisin microcrystals in hydrogel beads for controlled release applications. Eur. J. Pharm. Sci. 2007, 32, 17–23. [Google Scholar] [CrossRef]
- Nograles, N.; Abdullah, S.; Shamsudin, M.N.; Billa, N.; Rosli, R. Formation and characterization of pDNA-loaded alginate microspheres for oral administration in mice. J. Biosci. Bioeng. 2012, 113, 133–140. [Google Scholar] [CrossRef]
- Szekalska, M.; Amelian, A.; Winnicka, K. Alginate microspheres obtained by the spray drying technique as mucoadhesive carriers of ranitidine. Acta Pharm. 2015, 65, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fareez, I.M.; Lim, S.M.; Zulkefli, N.A.A.; Mishra, R.K.; Ramasamy, K. Cellulose derivatives enhanced stability of alginate-based beads loaded with Lactobacillus plantarum LAB12 against low pH, high temperature and prolonged storage. Probiotics Antimicrob. Proteins 2018, 10, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.-P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-encapsulation of probiotic Lactobacillus acidophilus and Reishi medicinal mushroom (Ganoderma lingzhi) extract in moist calcium alginate beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate nanoformulation: Influence of process and selected variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shang, Z.; Liu, H.; Yuan, Z.-X. Alginate-based platforms for cancer-targeted drug delivery. Biomed. Res. Int. 2020, 2020, 17. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [Green Version]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bhanjana, G.; Verma, R.K.; Dhingra, D.; Dilbaghi, N.; Kim, K.H. Metformin-loaded alginate nanoparticles as an effective antidiabetic agent for controlled drug release. J. Pharm. Pharmacol. 2017, 69, 143–150. [Google Scholar] [CrossRef]
- Thomas, D.; KurienThomas, K.; Latha, M.S. Preparation and evaluation of alginate nanoparticles prepared by green method for drug delivery applications. Int. J. Biol. Macromol. 2020, 154, 888–895. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Pharmacokinetic and pharmacodynamic behaviour of antitubercular drugs encapsulated in alginate nanoparticles at two doses. Int. J. Antimicrob. Agents 2006, 27, 409–416. [Google Scholar] [CrossRef]
- Bakhshi, M.; Ebrahimi, F.; Nazarian, S.; Zargan, J.; Behzadi, F.; Gariz, D.S. Nano-encapsulation of chicken immunoglobulin (IgY) in sodium alginate nanoparticles: In vitro characterization. Biologicals 2017, 17, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Andriamanantoanina, H.; Rinaudo, M. Relationship between the molecular structure of alginates and their gelation in acidic conditions. Polym. Int. 2010, 59, 1531–1541. [Google Scholar] [CrossRef]
- Rehm, B.H.; Valla, S. Bacterial alginates: Biosynthesis and applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.Y.; Wong, P.K.; Wong, C.L. Extraction and characterization of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar] [CrossRef]
- Abbah, S.A.; Lu, W.W.; Chan, D.; Cheung, K.M.; Liu, W.G.; Zhao, F.; Li, Z.Y.; Leong, J.C.; Luk, K.D. In vitro evaluation of alginate encapsulated adipose-tissue stromal cells for use as injectable bone graft substitute. Biochem. Biophys. Res. Commun. 2006, 347, 185–191. [Google Scholar] [CrossRef]
- Durrieu, M.C.; Pallu, S.; Guillemot, F.; Bareille, R.; Amedee, J.; Baquey, C.H.; Labrugère, C.; Dard, M. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J. Mater. Sci. Mater. Med. 2004, 15, 779–786. [Google Scholar] [CrossRef]
- Grellier, M.; Granja, P.L.; Fricain, J.C.; Bidarra, S.J.; Renard, M.; Bareille, R.; Bourget, C.; Amédée, J.; Barbosa, M.A. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials 2009, 30, 3271–3278. [Google Scholar] [CrossRef]
- Jin, H.H.; Kim, D.H.; Kim, T.W.; Shin, K.K.; Jung, J.S.; Park, H.C.; Yoon, S.Y. In vitro evaluation of porous hydroxyapatite/chitosan-alginate composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1079–1085. [Google Scholar] [CrossRef]
- Rubert, M.; Monjo, M.; Lyngstadaas, S.P.; Ramis, J.M. Effect of alginate hydrogel containing polyproline-rich peptides on osteoblast differentiation. Biomed. Mat. 2012, 7, 055003. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, W.; Weir, M.D.; Thein-Han, W.; Xu, H. Human embryonic stem cel encapsulation in alginate microbeads in macroporous calcium phosphate cement for bone tissue engineering. Acta Biomater. 2012, 8, 3436–3445. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Weir, M.D.; Bao, C.; Xu, H. Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012, 8, 2297–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Mei, F.; Duan, Y.; Gao, Y.; Xiong, Z.; Zhang, T.; Zhang, H. Bone tissue engineering using bone marrow stromal cells and an injectable sodium alginate/gelatin scaffold. J. Biomed. Mater. Res. A 2012, 100, 1044–1050. [Google Scholar] [CrossRef]
- Brun, F.; Turco, G.; Accardo, A.; Paoletti, S. Automated quantitative characterization of alginate/hydroxyapatite bone tissue engineering scaffolds by means of micro-CT image analysis. J. Mater. Sci. Mater. Med. 2011, 22, 2617–2629. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Leung, M.; Jana, S.; Li, Z.; Bhattarai, N.; Huang, J.; Hopper, R.A.; Zhang, M. Enhanced bone tissue formation by alginate gel-assisted cell seeding in porous ceramic scaffolds and sustained release of growth factor. J. Biomed. Mater. Res. A 2012, 100, 3408–3415. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.S.; Cho, J.; Tay, F.; Heng, J.Y.; Ho, R.; Kazarian, S.G.; Williams, D.R.; Boccaccini, A.R.; Polak, J.M.; Mantalaris, A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials 2009, 30, 499–507. [Google Scholar] [CrossRef]
- Fay, D.; Halsey, J. Anatomy of Bones; World Technologies: Bellingham, WA, USA, 2012. [Google Scholar]
- Lin, H.-R.; Yeh, Y.-J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Kim, D.J.; Wood, D.L.; Zhang, M. Influence of processing parameters on pore structure of 3D porous chitosan-alginate polyelectrolyte complex scaffolds. J. Biomed. Mater. Res. A 2011, 98, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 137, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.J. The use of gauze: Will it ever change? Int. Wound. J. 2006, 3, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, L.; Kennedy, J.F.; Methacanon, P.; Paterson, M.; Knill, C.J. Carbohydrate polymers as wound management aids. Carbohydr. Polym. 1998, 37, 315–322. [Google Scholar] [CrossRef]
- Bishop, S.M.; Walker, M.; Rogers, A.A.; Chen, W.Y. Importance of moisture balance at the wound-dressing interface. J. Wound Care. 2003, 12, 125–128. [Google Scholar] [CrossRef]
- Thomas, A.; Harding, K.G.; Moore, K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-α. Biomaterials 2000, 21, 1797–1802. [Google Scholar] [CrossRef]
- Walker, M.; Hobot, J.A.; Newman, G.R.; Bowler, P.G. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL®) and alginate dressings. Biomaterials 2003, 24, 883–890. [Google Scholar] [CrossRef]
- Miraftab, M.; Qiao, Q.; Kennedy, J.F.; Anand, S.C.; Groocock, M.R. Fibres for wound dressings based on mixed carbohydrate polymer fibres. Carbohydr. Polym. 2003, 53, 225–231. [Google Scholar] [CrossRef]
- Grothe, T.; Grimmelsmann, N.; Homburg, S.V.; Ehrmann, A. Possible applications of nano-spun fabrics and materials. Mater. Today Proc. 2017, 4, S154–S159. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Heinze, T.; Hipler, U.C. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009, 17, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S. Zinc in wound repair. Arch. Dermatol. 1999, 135, 1273–1274. [Google Scholar] [CrossRef]
- Wiśniewska-Wrona, M.; Kucharska, M.; Struszczyk, M.H.; Cichecka, M.; Wilbik-Hałgas, B.; Szymonowicz, M.; Paluch, D.; Guzińska, K.; Rybak, Z. Hemostatic, resorbable dressing of natural polymers-hemoguard. Autex Res. J. 2016, 16, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Zhang, M.; Zhao, F.; Gao, J.; Wang, L. Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing. E-Polymers 2019, 19, 268–276. [Google Scholar] [CrossRef]
- Dai, M.; Zheng, X.; Xu, X.; Kong, X.; Li, X.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z. Chitosan-alginate sponge: Preparation and application in curcumin delivery for dermal wound healing in rat. J. Biomed. Biotechnol. 2009, 2009, 8. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Khor, E.; Lim, L.Y. PEC films prepared from chitosan-alginate coacervates. Chem. Pharm. Bull. 2000, 48, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Venkatrajah, B.; Vanitha Malathy, V.; Elayarajah, B.; Mohan; Rajendran, R.; Rammohan, R. Biopolymer and Bletilla striata herbal extract coated cotton gauze preparation for wound healing. J. Med. Sci. 2012, 12, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Holak, P.; Adamiak, Z.; Babinska, I.; Jalynski, M.; Jastrzebski, P.; Grabarczyk, L.; Brzezinski, M.; Bory, J.; Tobolska, A.; Glodek, J. The influence of haemostatic dressing prototypes for the emergency services on the histopathological parameters of porcine muscle. In Vivo 2019, 33, 723–729. [Google Scholar] [CrossRef]
- Straccia, M.C.; d’Ayala, G.G.; Romano, L.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez Chabala, L.F.; Cuartas, C.E.E.; Lopez, M.E.L. Release behavior and antibacterial activity of chitosan/alginate blends with aloe vera and silver nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy Eldin, M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Kim, J.O.; Choi, J.Y.; Park, J.K.; Kim, J.H.; Jin, S.G.; Chang, S.W.; Li, D.X.; Hwang, M.-R.; Woo, J.S.; Kim, J.-A.; et al. Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol. Pharm. Bull. 2008, 31, 2277–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benselfelt, T.; Wågberg, L. Unidirectional swelling of dynamic cellulose nanofibril networks: A platform for tunable hydrogels and aerogels with 3D shapeability. Biomacromolecules 2019, 20, 2406–2412. [Google Scholar] [CrossRef]
- Kurczewska, J.; Pecyna, P.; Ratajczak, M.; Gajecka, M.; Schroeder, G. Halloysite nanotubes as carriers of vancomycin in alginate-based wound dressing. Saudi Pharm. J. 2017, 25, 911–920. [Google Scholar] [CrossRef]
- Kumar, L.; Brice, J.; Toberer, L.; Klein-Seetharaman, J.; Knauss, D.; Sarkar, S.K. Antimicrobial biopolymer formation from sodium alginate and algae extract using aminoglycosides. PLoS ONE 2019, 14, 1492. [Google Scholar] [CrossRef]
- Osmokrović, A.; Jančić, I.; Jankovic-Castvan, I.; Petrović, P.; Milenkovic, M.; Obradovic, B. Novel composite zinc-alginate hydrogels with activated charcoal aimed for potential applications in multifunctional primary wound dressings. Hem. Ind. 2019, 73, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Hong, G.; Kwon, T.; Lim, J. Fabrication of oxygen releasing scaffold by embedding H2O2-PLGA microspheres into alginate-based hydrogel sponge and its application for wound healing. Appl. Sci. 2018, 8, 1492. [Google Scholar] [CrossRef] [Green Version]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin-a review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Xia, S.; Cai, J.; Zhang, X.; Duhoranimana, E. Gelatin and pectin complex coacervates as carriers for cinnamaldehyde: Effect of pectin esterification degree on coacervate formation, and enhanced thermal stability. Food Hydrocolloid. 2019, 87, 712–722. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khramova, D.S.; Vityazev, F.V.; Saveliev, N.Y.; Burkov, A.A.; Beloserov, V.S.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Pectin gelling in acidic gastric condition increases rheological properties of gastric digesta and reduces glycaemic response in mice. Carbohydr. Polym. 2019, 205, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, M.; Chandra Atluri, S.; Chen, J.; Gilbert, R.G. Relations between digestibility and structures of pumpkin starches and pectins. Food Hydrocoll. 2020, 106, 105894. [Google Scholar] [CrossRef]
- Zhu, R.-G.; Sun, Y.-D.; Li, T.-P.; Chen, G.; Peng, X.; Duan, W.-B.; Zheng, Z.-Z.; Shi, S.-L.; Xu, J.-G.; Liu, Y.-H.; et al. Comparative effects of hawthorn (Crataegus pinnatifida Bunge) pectin and pectin hydrolyzates on the cholesterol homeostasis of hamsters fed high-cholesterol diets. Chem. Biol. Interact. 2015, 238, 42–47. [Google Scholar] [CrossRef]
- Zhu, R.-G.; Sun, Y.-D.; Hou, Y.-T.; Fan, J.-G.; Chen, G.; Li, T.-P. Pectin penta-oligogalacturonide reduces cholesterol accumulation by promoting bile acid biosynthesis and excretion in high-cholesterol-fed mice. Chem. Biol. Interact. 2017, 272, 153–159. [Google Scholar] [CrossRef]
- Li, Y.; Niu, Y.; Wu, H.; Sun, Y.; Li, Q.; Kong, X.; Liu, L.; Mei, Q. Modified apple polysaccharides could induce apoptosis in colorectal cancer cells. J. Food Sci. 2010, 75, H224–H229. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Djaldetti, M.; Salman, H.; Bessler, H. Effect of citrus pectin on malignant cell proliferation. Biomed. Pharmacother. 2010, 64, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods. 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Madziva, H.; Kailasapathy, K.; Philips, M. Alginate-pectin microcapsules as a potential for folic acid delivery in foods. J. Microencapsul. 2005, 22, 343–351. [Google Scholar] [CrossRef]
- Pour, P.K.; Alezmadeh, I.; Vaziri, A.S.; Beiroti, A. Potential effects of alginate-pectin biocomposite on the release of folic acid and their physicochemical characteristics. J. Food Sci. Technol. 2020, 57, 3363–3370. [Google Scholar] [CrossRef]
- Islan, G.A.; de Verti, I.P.; Marchetti, S.G.; Castro, G.R. Studies of ciprofloxacin encapsulation on alginate/pectin matrixes and its relationship with biodisponibility. Appl. Biochem. Biotechnol. 2012, 167, 1408–1420. [Google Scholar] [CrossRef]
- Daas, P.J.H.; Boxma, B.; Hopman, A.M.C.P.; Voragen, A.G.J.; Schols, H.A. Nonesterified galacturonic acid sequence homology of pectins. Biopolymers 2001, 58, 1–8. [Google Scholar] [CrossRef]
- Asai, J.; Takenaka, H.; Hirakawa, S.; Sakabe, J.; Hagura, A.; Kishimoto, S.; Maruyama, K.; Kajiya, K.; Kinoshita, S.; Tokura, Y.; et al. Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am. J. Pathol. 2012, 181, 2217–2224. [Google Scholar] [CrossRef]
- Adami, M.; Prudente, A.S.; Mendes, D.A.; Horinouchi, C.D.; Cabrini, D.A.; Otuki, M.F. Simvastatin ointment, a new treatment for skin inflammatoryconditions. J. Dermatol. Sci. 2012, 66, 127–135. [Google Scholar] [CrossRef]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Liu, C.M.; He, X.H.; Liang, R.H.; Liu, W.; Guo, W.L.; Chen, J. Relating physicochemical properties of alginate-HMP complexes to their performace as drug delivery systems. J. Biomater. Sci. Polym. Ed. 2017, 28, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.W.; Nam, S.Y.; Heo, S.J.; Kang, D.H.; Jung, W.K. Characterization of ionic cross-linked composite foams with different blend ratios of alginate/pectin on the synergistic effects for wound dressing application. Int. J. Biol. Macromol. 2020, 156, 1565–1573. [Google Scholar] [CrossRef]
- Awasthi, R.; Kulkarni, G.T. Development of novel gastroretentive drug delivery system of gliclazide: Hollow beads. Drug Dev. Ind. Pharm. 2014, 40, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Jelvehgari, M.; Mobaraki, V.; Montazam, S.H. Preparation and evaluation of mucoadhesive beads/discs of alginate and algino-pectinate of piroxicam for colon-specific drug delivery via oral route. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e16576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, R.; Kulkarni, G.T.; Ramana, M.V.; de Jesus Andreoli Pinto, T.; Kikuchi, I.S.; Molim Ghisleni, D.D.; de Souza Braga, M.; De Bank, P.; Dua, K. Dual crosslinked pectin–alginate network as sustained release hydrophilic matrix for repaglinide. Int. J. Biol. Macromol. 2017, 97, 721–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 123, 112009. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry, 1st ed.; The Mac Millan Press: London, UK, 1992; pp. 274–315. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, R.; Reader, S.; Falshaw, A. Chitosan functional properties. Glycoconj. J. 1997, 14, 535–542. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Shi, Y.; Zhao, L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv. Transl. Res. 2019, 9, 227–239. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release. 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Wang, X.W.; Zhang, T.; Shi, B.H.; Zheng, B.; Zhang, D.D.; Hu, Y.Y.; Ma, L.; et al. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2020, 28, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Caetano, G.F.; Frade, M.A.; Andrade, T.A.; Leite, M.N.; Bueno, C.Z.; Moraes, Â.M.; Ribeiro-Paes, J.T. Chitosan-alginate membranes accelerate wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- D’Ayala, G.G.; De Rosa, A.; Laurienzo, P.; Malinconico, M. Development of a new calcium sulphate-based composite using alginate and chemically modified chitosan for bone regeneration. J. Biomed. Mater. Res. A 2007, 81, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef]
- Lacerda, L.; Parize, A.L.; Fávere, V.; Laranjeira, M.C.; Stulzer, H.K. Development and evaluation of pH-sensitive sodium alginate/chitosan microparticles containing the antituberculosis drug rifampicin. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 39, 161–167. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Liu, L.; Li, M.; Zuo, A.; Guo, J. Preparation, in vitro and in vivo evaluation of chitosan-sodium alginate-ethyl cellulose polyelectrolyte film as a novel buccal mucosal delivery vehicle. Eur. J. Pharm. Sci. 2022, 168, 106085. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Saladini, B.; Gallucci, M.C.; Cruciani, F.; Vitali, B.; Luppi, B. Chitosan/alginate complexes for vaginal delivery of chlorhexidine digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef]
- Digenis, G.A.; Gold, T.B.; Shah, V.P. Cross-linking of gelatin capsules and its relevance to their in vitro-in vivo performance. J. Pharm. Sci. 1994, 83, 915–921. [Google Scholar] [CrossRef]
- Lopes, S.; Bueno, L.; Aguiar, F., Jr.; Finkler, C. Preparation and characterization of alginate and gelatin microcapsules containing Lactobacillus rhamnosus. An. Acad. Bras. Ciênc. 2017, 89, 1601–1613. [Google Scholar] [CrossRef]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences; Prasad, R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 330–352. [Google Scholar] [CrossRef]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Vandelli, M.A.; Romagnoli, M.; Monti, A.; Gozzi, M.; Guerra, P.; Rivasi, F.; Forni, F. Microwave-treated gelatin microspheres as drug delivery system. J. Control. Release. 2004, 96, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Raymond, G.; Degennaro, M.; Mikeal, R. Preparation of gelatin: Phenytoin sodium microsphers: An in vitro and in vivo Evaluation. Drug Dev. Ind. Pharm. 1990, 16, 1025–1051. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Cross-linking of gelatin with carbodiimides. Tissue Eng. 1996, 2, 307–313. [Google Scholar] [CrossRef]
- Nezhadi, S.H.; Choong, P.F.; Lotfipour, F.; Dass, C.R. Gelatin-based delivery systems for cancer gene therapy. J. Drug Target. 2009, 17, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Zhang, R.; Luan, J.; Lin, F. Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication 2012, 4, 025007. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Morshedloo, F.; Khoshfetrat, A.B.; Kazemi, D.; Ahmadian, M. Gelatin improves peroxidase-mediated alginate hydrogel characteristics as a potential injectable hydrogel for soft tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2950–2960. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [Green Version]
- Leone, G.; Consumi, M.; Pepi, S.; Lamponi, S.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Alginate–gelatin formulation to modify lovastatin release profile from red yeast rice for hypercholesterolemia therapy. Ther. Deliv. 2017, 8, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Childress, L.; Gay, A.; Zargar, A.; Ito, M.K. Review of red yeast rice content and current Food and Drug Administration oversight. J. Clin. Lipidol. 2013, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110957. [Google Scholar] [CrossRef]
- Bidgoli, M.R.; Alemzadeh, I.; Tamjid, E.; Khafaji, M.; Vossoughi, M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: Effect of particle size on physico-mechanical properties and in vitro cellular behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109688. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G. Biomechanics of soft tissue. In The Handbook of Materials Behavior Models; Composite Media; Lemaitre, J., Ed.; Academic Press: Boston, MA, USA, 2001; Volume 3, pp. 1057–1071. [Google Scholar] [CrossRef]
- Türe, H. Characterization of hydroxyapatite-containing alginate–gelatin composite films as a potential wound dressing. Int. J. Biol. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K. Carrageenan. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; pp. 122–126. [Google Scholar]
- Gupta, V.K.; Hariharan, M.; Wheatley, T.A.; Price, J.C. Controlled-release tablets from carrageenans: Effect of formulation, storage and dissolution factors. Eur. J. Pharm. Biopharm. 2001, 51, 241–248. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.-P.; Martín-Illana, A.; Ruiz-Caro, R.; Bermejo, P.; Abad, M.-J.; Carro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Fernández-Ferreiro, A.; et al. Chitosan and Kappa-Carrageenan Vaginal Acyclovir Formulations for Prevention of Genital Herpes. In Vitro and Ex Vivo Evaluation. Mar. Drugs. 2015, 13, 5976–5992. [Google Scholar] [CrossRef] [Green Version]
- Zaveri, T.; Hayes, J.; Ziegler, G. Release of tenofovir from carrageenan-based vaginal suppositories. Pharmaceutics 2014, 6, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Bani-Jaber, A.; Abdullah, S. Development and characterization of novel ambroxol sustained-release oral suspensions based on drug-polymeric complexation and polymeric raft formation. Pharm. Dev. Technol. 2020, 25, 666–675. [Google Scholar] [CrossRef]
- Salgueiro, A.M.; Daniel-da-Silva, A.L.; Fateixa, S.; Trindade, T. κ-Carrageenan hydrogel nanocomposites with release behavior mediated by morphological distinct Au nanofillers. Carbohydr. Polym. 2013, 91, 100–109. [Google Scholar] [CrossRef]
- Kailasapathy, K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr. Issues. Intest. Microbiol. 2002, 3, 39–48. [Google Scholar] [PubMed]
- Jonganurakkun, B.; Nodasaka, Y.; Sakairi, N.; Nishi, N. DNA-based gels for oral delivery of probiotic bacteria. Macromol. Biosci. 2006, 6, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozsoy, Y.; Bergişadi, N. Preparation of mefenamic acid sustained release beads based on kappa-carrageenan. Boll. Chim. Farm. 2000, 139, 120–123. [Google Scholar] [PubMed]
- Sipahigil, O.; Dortunç, B. Preparation and in vitro evaluation of verapamil HCl and ibuprofen containing carrageenan beads. Int. J. Pharm. 2001, 228, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Caramella, C.; Díez-Pérez, I.; Gorostiza, P.; Lastra, C.; Oliva, I.; Mariño, E. Sequential atomic force microscopy imaging of a spontaneous nanoencapsulation process. Int. J. Pharm. 2002, 242, 291–294. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Inhibitory action of natural carrageenans on Herpes simplex virus infection of mouse astrocytes. Chemotherapy. 1999, 45, 429–436. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of Papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- González, M.E.; Alarcón, B.; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, G.R.; Rahmani, Z.; Karami, S.; Pourjavadi, A. Magnetic/pH-sensitive κ-carrageenan/sodium alginate hydrogel nanocomposite beads: Preparation, swelling behavior, and drug delivery. J. Biomater. Sci. Polym. Ed. 2014, 25, 1891–1906. [Google Scholar] [CrossRef]
- Akiyode, O.; Boateng, J. Composite biopolymer-based wafer dressings loaded with microbial biosurfactants for potential application in chronic wounds. Polymers 2018, 10, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zia, T.; Usman, M.; Sabir, A.; Shafiq, M.; Khan, R.U. Development of inter-polymeric complex of anionic polysaccharides, alginate/k-carrageenan bio-platform for burn dressing. Int. J. Biol. Macromol. 2020, 157, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Silva Batalha, L.; Pardini Gontijo, M.T.; Vianna Novaes de Carvalho Teixeira, A.; Meireles Gouvêa Boggione, D.; Soto Lopez, M.E.; Renon Eller, M.; Santos Mendonça, R.C. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef]
- Qi, X.; Simsek, S.; Chen, B.; Rao, J. Alginate-based double-network hydrogel improves the viability of encapsulated probiotics during simulated sequential gastrointestinal digestion: Effect of biopolymer type and concentrations. Int. J. Biol. Macromol. 2020, 165, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.W.; Jung, W.K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.T.; Zhang, Q.; Wang, B.; Zhang, T. Formulation development and evaluation of gastroretentive floating beads with Brucea javanica oil using ionotropic gelation technology. Chin. J. Nat. Med. 2018, 16, 293–301. [Google Scholar] [CrossRef]









| ALG Derivative | Physical Form | Water Solubility | Pharmaceutical Applications |
|---|---|---|---|
| Alginic acid | Odorless and tasteless, white to yellowish fibrous powder | Swells in water without dissolving | Binder and disintegrating agent (1–5%) in tablet technology; thickening and suspending agent in semi-solid drug forms |
| Sodium alginate | Odorless, tasteless, white to pale yellow-brown powder | Good water solubility with forming viscous solutions | Binder and disintegration agent in tablet technology in sustained-release dosage forms; thickening and suspending agent in o/w emulsions, creams, pastes; in microparticles and nanoparticles technology; mucoadhesive dosage forms |
| Calcium alginate | Odorless, tasteless, white to pale yellow-brown powder or fibers | Practically insoluble in water | Tablet disintegrant, wound dressings, floating dosage systems, dosage forms with modified release |
| Ammonium alginate | White to yellow-brown threaded, granular or powdered forms | Slowly dissolves in water | Emulsifying agent, film-former, humectant |
| Propylene glycol alginate | Practically odorless and tasteless white to yellowish granular or fibrous powder | Soluble in water mixed with ethanol 95% up to 60% (w/w) (depending on esterification degree) | Emulsifying, stabilizing, gelling and suspending agent |
| ALG Pharmaceutical Applications | Reference |
|---|---|
| Semi-solid dosage forms | [1,8] |
| Microparticles | [91,92,93,94,95,96,97,98] |
| Mucoadhesive dosage forms | [90,102] |
| Nanoparticles | [109,110,111,112] |
| Tablets technology | [113,114,115] |
| Tissue engineering | [118,130] |
| Bone and cartilage regeneration | [120,121] |
| Wound dressing | [138,139] |
| Cell culture | [124,125,126,127,128,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, K.; Winnicka, K. Alginates Combined with Natural Polymers as Valuable Drug Delivery Platforms. Mar. Drugs 2023, 21, 11. https://doi.org/10.3390/md21010011
Kruk K, Winnicka K. Alginates Combined with Natural Polymers as Valuable Drug Delivery Platforms. Marine Drugs. 2023; 21(1):11. https://doi.org/10.3390/md21010011
Chicago/Turabian StyleKruk, Katarzyna, and Katarzyna Winnicka. 2023. "Alginates Combined with Natural Polymers as Valuable Drug Delivery Platforms" Marine Drugs 21, no. 1: 11. https://doi.org/10.3390/md21010011