Synthesis of Water-Soluble Sulfonated Chitin Derivatives for Potential Antioxidant and Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Chitin Derivatives
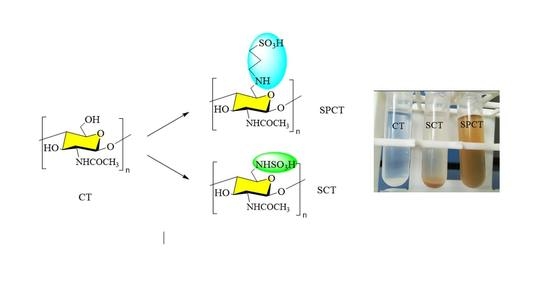
2.1.1. Synthesis of Chitin Derivatives
2.1.2. FTIR Analysis
2.1.3. NMR Analysis
2.1.4. Thermogravimetric and Derivative Thermogravimetric Analysis (TGA/DTG)
2.1.5. X-ray Diffraction (XRD) Analysis
2.2. Antioxidant Activities
2.2.1. DPPH-Radical Scavenging Ability Assay
2.2.2. Hydroxyl-Radical Scavenging Ability Assay
2.2.3. Superoxide-Radical Scavenging Ability Assay
2.2.4. Reducing Power Assay
2.3. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthesis
3.3.1. Preparation of Sulfo-Chitin (SCT)
3.3.2. Preparation of Sulfopropyl-Chitin (SPCT)
3.4. Investigation of the Antioxidant Activity
3.4.1. DPPH-Radical Scavenging Activity
3.4.2. Hydroxyl-Radical Scavenging Activity
3.4.3. Superoxide-Radical Scavenging Activity
3.4.4. Reducing Power Activity
3.5. Evaluation of Antifungal Activity In Vitro
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, H.; Yin, L.; Hu, R.; Qiu, T.; Yin, Y.; Xiong, X.; Zheng, H.; Wang, Q. A pH-Sensitive Nanosystem Based on Carboxymethyl Chitosan for Tumor-Targeted Delivery of Daunorubicin. J. Biomed. Nanotechnol. 2016, 12, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zheng, H.; Cao, J.; Davoudi, Z.; Wang, Q. Synthesis and In Vitro Characterization of Carboxymethyl Chitosan-CBA-Doxorubicin Conjugate Nanoparticles as pH-Sensitive Drug Delivery Systems. J. Biomed. Nanotechnol. 2017, 13, 1097–1105. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef]
- Wei, X.; Liao, J.; Davoudi, Z.; Zheng, H.; Chen, J.; Li, D.; Xiong, X.; Yin, Y.; Yu, X.; Xiong, J.; et al. Folate Receptor-Targeted and GSH-Responsive Carboxymethyl Chitosan Nanoparticles Containing Covalently Entrapped 6-Mercaptopurine for Enhanced Intracellular Drug Delivery in Leukemia. Mar. Drugs 2018, 16, 439. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Chen, X. Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of marine-derived biowaste to develop chitin/fish gelatin products as bioactive carriers and moisture scavengers. Sci. Total Environ. 2020, 706, 135747. [Google Scholar] [CrossRef]
- Kertmen, A.; Ehrlich, H. Patentology of chitinous biomaterials. Part I: Chitin. Carbohydr. Polym. 2022, 282, 119102. [Google Scholar] [CrossRef]
- Li, F.; You, X.; Li, Q.; Qin, D.; Wang, M.; Yuan, S.; Chen, X.; Bi, S. Homogeneous deacetylation and degradation of chitin in NaOH/urea dissolution system. Int. J. Biol. Macromol. 2021, 189, 391–397. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Zeng, F.; Jia, Q.; Zhang, L.; Yu, A.; Duan, B. A quaternized chitin derivatives, egg white protein and montmorillonite composite sponge with antibacterial and hemostatic effect for promoting wound healing. Compos. Part B Eng. 2022, 234, 109661. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seedevi, P.; Moovendhan, M.; Vairamani, S.; Shanmugam, A. Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int. J. Biol. Macromol. 2017, 99, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Dimassi, S.; Tabary, N.; Leclercq, L.; Degoutin, S.; Chai, F.; Pierlot, C.; Cazaux, F.; Ung, A.; Staelens, J.N.; et al. Synthesis and characterization of polyampholytic aryl-sulfonated chitosans and their in vitro anticoagulant activity. Carbohydr. Polym. 2018, 196, 8–17. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles. Bioorg. Chem. 2020, 94, 103396. [Google Scholar]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, L.; Hussain, Z.; Huang, D.; Zhu, S. Enhancement of storability and antioxidant systems of sweet cherry fruit by nitric oxide-releasing chitosan nanoparticles (GSNO-CS NPs). Food Chem. 2019, 285, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Zhu, Z.; Sun, X.; Yuan, Z.; Zha, J.; Wen, Y. Highly efficient antifogging and antibacterial food packaging film fabricated by novel quaternary ammonium chitosan composite. Food Chem. 2020, 308, 125682. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch-Stärke 2021, 73, 2000218. [Google Scholar] [CrossRef]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Vikhoreva, G.; Bannikova, G.; Stolbushkina, P.; Panov, A.; Drozd, N.; Makarov, V.; Varlamov, V.; Galbraikh, L. Preparation and anticoagulant activity of a low-molecular-weight sulfated chitosan. Carbohydr. Polym. 2005, 62, 327–332. [Google Scholar] [CrossRef]
- Jeon, J.H.; Cheedarala, R.K.; Kee, C.D.; Oh, I.K. Dry-Type Artificial Muscles Based on Pendent Sulfonated Chitosan and Functionalized Graphene Oxide for Greatly Enhanced Ionic Interactions and Mechanical Stiffness. Adv. Funct. Mater. 2013, 23, 6007–6018. [Google Scholar] [CrossRef]
- Tsai, H.S.; Wang, Y.Z.; Lin, J.J.; Lien, W.F. Preparation and properties of sulfopropyl chitosan derivatives with various sulfonation degree. J. Appl. Polym. Sci. 2009, 116, 1686–1693. [Google Scholar] [CrossRef]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.M.; Fouda, M.M.; Soliman, A.Y.; Mohamed, F.K.; Mohsin, K.; Pinto, T.D. Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Wang, P.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of N-Quaternized and N-Diquaternized Chitin Derivatives. Starch-Stärke 2018, 70, 1800026. [Google Scholar] [CrossRef]
- Cai, J.; Dang, Q.; Liu, C.; Wang, T.; Fan, B.; Yan, J.; Xua, Y. Preparation, characterization and antibacterial activity of O-acetyl-chitosan-N-2-hydroxypropyl trimethyl ammonium chloride. Int. J. Biol. Macromol. 2015, 80, 8–15. [Google Scholar] [CrossRef]
- Shahzad, S.; Shahzadi, L.; Mahmood, N.; Siddiqi, S.A.; Rauf, A.; Manzoor, F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. A new synthetic methodology for the preparation of biocompatible and organo-soluble barbituric- and thiobarbituric acid based chitosan derivatives for biomedical applications. Mater. Sci. Eng. C 2016, 66, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Crystallinity and hydrophility of chitin and chitosan. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Duh, P.D.; Du, P.C.; Yen, G.C. Action of Methanolic Extract of Mung Bean Hulls as Inhibitors of Lipid Peroxidation and Non-lipid Oxidative Damage. Food Chem. Toxicol. 1999, 37, 1055–1061. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Sun, X.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and Antioxidant Activity of Cationic 1,2,3-Triazole Functionalized Starch Derivatives, Polymers. Polymers 2020, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Saesoo, S.; Ovatlarnporn, C. Antifungal property of quaternized chitosan and its derivatives. Int. J. Biol. Macromol. 2012, 50, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luan, F.; Li, Q.; Gu, G.; Dong, F.; Guo, Z. Synthesis of Novel Chitin Derivatives Bearing Amino Groups and Evaluation of Their Antifungal Activity. Mar. Drugs 2018, 16, 380. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Tan, W.; Zhang, J.; Guo, Z. Modification of Hydroxypropyltrimethyl Ammonium Chitosan with Organic Acid: Synthesis, Characterization, and Antioxidant Activity. Polymers 2020, 12, 2460. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Chen, Y.; Mi, Y.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts. Polymers 2019, 11, 1810. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhang, X.; Gao, X.; Meng, X.; Yi, Y. Synthesis and Characterization of Inulin Butyrate Ester, and Evaluation of Its Antioxidant Activity and In Vitro Effect on SCFA Production. Starch-Stärke 2020, 72, 1900323. [Google Scholar] [CrossRef]
- Luan, F.; Li, Q.; Tan, W.; Wei, L.; Zhang, J.; Dong, F.; Gu, G.; Guo, Z. The evaluation of antioxidant and antifungal properties of 6-amino-6-deoxychitosan in vitro. Int. J. Biol. Macromol. 2018, 107, 595–603. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Mi, Y.; Luan, F.; Wei, L.; Li, Q.; Dong, F.; Guo, Z. Synthesis and Characterization of Inulin Derivatives Bearing Urea Groups with Promising Antifungal Activity. Starch-Stärke 2019, 71, 1800058. [Google Scholar] [CrossRef]







| Full Name | Sample (Abbreviations) | Found (%) | DS | Formula | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| chitin | CT | 45.23 | 7.19 | 6.59 | 1.0 | C8H13NO5 | |
| tosyl-chitin | TCT | 50.02 | 6.56 | 4.53 | 7.86 | 0.93 | (C15H19NO7S)0.89(CT)0.11 |
| azido-chitin | ACT | 44.25 | 5.22 | 20.87 | 0.90 | (TCT)0.1 (C8H12N4O4)0.9 | |
| amino-chitin | NCT | 46.95 | 6.80 | 14.97 | 0.87 | (ACT)0.04 (C8H14N2O4)0.87 | |
| sulfo-chitin | SCT | 36.90 | 5.85 | 9.84 | 8.74 | 0.40 | (NCT)0.6(C8H14N2O7S)0.4 |
| sulfopropyl-chitin | SPCT | 39.62 | 6.11 | 9.88 | 8.14 | 0.41 | (NCT)0.59(C11H19N2O7S)0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, F.; Xu, Z.; Wang, K.; Qi, X.; Guo, Z. Synthesis of Water-Soluble Sulfonated Chitin Derivatives for Potential Antioxidant and Antifungal Activity. Mar. Drugs 2022, 20, 668. https://doi.org/10.3390/md20110668
Luan F, Xu Z, Wang K, Qi X, Guo Z. Synthesis of Water-Soluble Sulfonated Chitin Derivatives for Potential Antioxidant and Antifungal Activity. Marine Drugs. 2022; 20(11):668. https://doi.org/10.3390/md20110668
Chicago/Turabian StyleLuan, Fang, Zhenhua Xu, Kai Wang, Xin Qi, and Zhanyong Guo. 2022. "Synthesis of Water-Soluble Sulfonated Chitin Derivatives for Potential Antioxidant and Antifungal Activity" Marine Drugs 20, no. 11: 668. https://doi.org/10.3390/md20110668