Design and Synthesis of New 4-(3,4,5-Trimethoxyphenyl)Thiazole–Pyrimidine Derivatives as Potential Antiproliferative Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Procedure for the Synthesis of 2-Bromo-1-(3,4,5-trimethoxyphenyl)ethan-1-one (1)
2.3. Procedure for the Synthesis of 4-(3,4,5-Trimethoxyphenyl)thiazol-2-amine (2)
2.4. Procedure for the Synthesis of N-(6-Chloro-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (3)
2.5. General Procedure for the Synthesis of N-(6-Substituted-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazole-2-amine (4a–4n)
2.5.1. N-(6-(4-Ethylpiperazin-1-yl)-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (4a)
2.5.2. 2-(4-(2-Methyl-6-((4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)amino)pyrimidin-4-yl)piperazin-1-yl)ethan-1-ol (4b)
2.5.3. N-(6-(4-(4-Methoxyphenyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (4c)
2.5.4. N-(6-(4-(4-Fluorophenyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (4d)
2.5.5. N-(6-(4-(4-Chlorophenyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (4e)
2.5.6. N-(6-(4-(2,3-Dichlorophenyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (4f)
2.5.7. N-(2-Methyl-6-morpholinopyrimidin-4-yl)-4-(3,4,5-trimethoxyphenyl)thiazol-2-amine (4g)
2.5.8. 2-Methyl-N4-(2-(4-methylpiperazin-1-yl)ethyl)-N6-(4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)pyrimidine-4,6-diamine (4h)
2.5.9. 2-Methyl-N4-(2-morpholinoethyl)-N6-(4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)pyrimidine-4,6-diamine (4i)
2.5.10. 2-((2-Methyl-6-((4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)amino)pyrimidin-4-yl)amino)ethan-1-ol (4j)
2.5.11. 2-Methyl-N4-(3-(4-methylpiperazin-1-yl)propyl)-N6-(4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)pyrimidine-4,6-diamine (4k)
2.5.12. 2-Methyl-N4-(3-morpholinopropyl)-N6-(4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)pyrimidine-4,6-diamine2 (4l)
2.5.13. N4-(3-(Dimethylamino)propyl)-2-methyl-N6-(4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)pyrimidine-4,6-diamine (4m)
2.5.14. 3-((2-Methyl-6-((4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)amino)pyrimidin-4-yl)amino)propan-1-ol (4n)
2.6. NCI-60 Screening
2.7. In Vitro Anticancer Screening (MTT Assay)
3. Results and Discussion
3.1. Chemistry
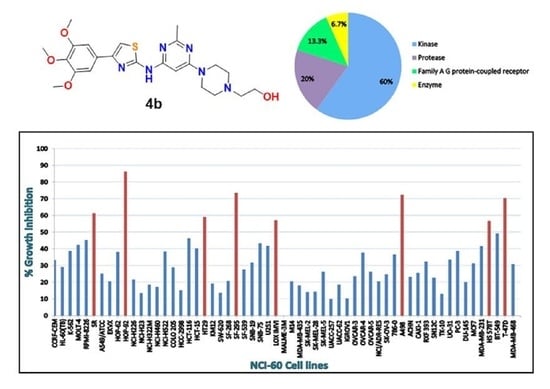
3.2. Antiproliferative Activities against NCI-60 Cell Line Panel at Single Dose Testing
3.3. In Vitro Anticancer MTT Assay against Four Cell Lines
3.4. Structure Similarity Search
3.5. Drug-Likeness and ADMET Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Cheng, Y.J.; Lei, Q.; Zhang, A.Q.; Zhang, X.Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef]
- Li, L.; Jiang, S.; Li, X.; Liu, Y.; Su, J.; Chen, J. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2018, 151, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Song, I.H.; Warkad, S.D.; Song, K.S.; Yeom, G.S.; Saha, S.; Shinde, P.B.; Nimse, S.B. Indazole-based microtubule-targeting agents as potential candidates for anticancer drugs discovery. Bioorg. Chem. 2022, 122, 105735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Kanakkanthara, A. Beyond the paclitaxel and vinca alkaloids: Next generation of plant-derived microtubule-targeting agents with potential anticancer activity. Cancers 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Li, N.; Guan, Q.; Hong, Y.; Zhang, B.; Li, M.; Li, X.; Li, B.; Wu, L.; Zhang, W. Discovery of 6-aryl-2-(3,4,5-trimethoxyphenyl) thiazole [3,2-b][1,2,4]triazoles as potent tubulin polymerization inhibitors. Eur. J. Med. Chem. 2023, 256, 115402. [Google Scholar] [CrossRef]
- Tian, C.; Wang, M.; Shi, X.; Chen, X.; Wang, X.; Zhang, Z.; Liu, J. Discovery of (2-(pyrrolidin-1-yl)thieno[3,2-d]pyrimidin-4-yl)(3,4,5-trimethoxyphenyl)methanone as a novel potent tubulin depolymerizing and vascular disrupting agent. Eur. J. Med. Chem. 2022, 238, 114466. [Google Scholar] [CrossRef]
- Hao, S.Y.; Qi, Z.Y.; Wang, S.; Wang, X.R.; Chen, S.W. Synthesis and bioevaluation of N-(3,4,5-trimethoxyphenyl)-1H-pyrazolo[3,4-b]pyridin-3-amines as tubulin polymerization inhibitors with anti-angiogenic effects. Bioorg. Med. Chem. 2021, 31, 115985. [Google Scholar] [CrossRef]
- Liu, R.; Huang, M.; Zhang, S.; Li, L.; Li, M.; Sun, J.; Wu, L.; Guan, Q.; Zhang, W. Design, synthesis and bioevaluation of 6-aryl-1-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazoles as tubulin polymerization inhibitors. Eur. J. Med. Chem. 2021, 226, 113826. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, M.; Zhou, P.; Liu, M.; Li, J.; Wang, Y. Design, synthesis and antitumor evaluation of novel chiral diaryl substituted azetidin-2-one derivatives as tubulin polymerization inhibitors. Bioorg. Chem. 2021, 115, 105239. [Google Scholar] [CrossRef] [PubMed]
- Zaki, I.; Abou-Elkhair, R.A.; Abu Almaaty, A.H.; Abu Ali, O.A.; Fayad, E.; Ahmed Gaafar, A.G.; Zakaria, M.Y. Design and synthesis of newly synthesized acrylamide derivatives as potential chemotherapeutic agents against MCF-7 breast cancer cell line lodged on PEGylated bilosomal nano-vesicles for improving cytotoxic activity. Pharmaceuticals 2021, 14, 1021. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ren, Y.; Pan, W.; Liu, J.; Chen, J. Discovery of novel acridane-based tubulin polymerization inhibitors with anticancer and potential immunomodulatory effects. J. Med. Chem. 2022, 66, 627–640. [Google Scholar] [CrossRef]
- Puxeddu, M.; Wu, J.; Bai, R.; D’Ambrosio, M.; Nalli, M.; Coluccia, A.; Manetto, S.; Ciogli, A.; Masci, D.; Urbani, A.; et al. Induction of Ferroptosis in Glioblastoma and Ovarian Cancers by a New Pyrrole Tubulin Assembly Inhibitor. J. Med. Chem. 2022, 65, 15805–15818. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Brancale, A.; Ricci, A.; Hamel, E.; Bortolozzi, R.; Basso, G.; Viola, G. Convergent synthesis and biological evaluation of 2-amino-4-(3,4,5-trimethoxyphenyl)-5-aryl thiazoles as microtubule targeting agents. J. Med. Chem. 2011, 54, 5144–5153. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, R.; Baraldi, P.G.; Salvador, M.K.; Camacho, M.E.; Preti, D.; Tabrizi, M.A.; Bassetto, M.; Brancale, A.; Hamel, E.; Bortolozzi, R.; et al. Synthesis and biological evaluation of 2-substituted-4-(3,4,5-trimethoxyphenyl)-5-aryl thiazoles as anticancer agents. Bioorg. Med. Chem. 2012, 20, 7083–7094. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Yang, Z.; Liu, Y.; Ma, L.; Wu, Y.; He, L.; Shao, M.; Yu, K.; Wu, W.; Pu, Y.; et al. Synthesis and biological evaluation of diarylthiazole derivatives as antimitotic and antivascular agents with potent antitumor activity. Bioorg. Med. Chem. 2015, 23, 3337–3350. [Google Scholar] [CrossRef]
- El-Abd, A.O.; Bayomi, S.M.; El-Damasy, A.K.; Mansour, B.; Abdel-Aziz, N.I.; El-Sherbeny, M.A. Synthesis and Molecular Docking Study of New Thiazole Derivatives as Potential Tubulin Polymerization Inhibitors. ACS Omega 2022, 7, 33599–33613. [Google Scholar] [CrossRef]
- Steinberg, M. Dasatinib: A tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome—Positive acute lymphoblastic leukemia. Clin. Ther. 2007, 29, 2289–2308. [Google Scholar] [CrossRef]
- Sabry, M.A.; Ghaly, M.A.; Maarouf, A.R.; El-Subbagh, H.I. New thiazole-based derivatives as EGFR/HER2 and DHFR inhibitors: Synthesis, molecular modeling simulations and anticancer activity. Eur. J. Med. Chem. 2022, 241, 114661. [Google Scholar] [CrossRef]
- Schade, A.E.; Schieven, G.L.; Townsend, R.; Jankowska, A.M.; Susulic, V.; Zhang, R.; Szpurka, H.; Maciejewski, J.P. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood Am. J. Hematol. 2008, 111, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarapu, L.; Vanaparthi, S.; Bantu, R.; Kumar, C.G. Synthesis of novel benzo [4,5] thiazolo [1,2-a] pyrimidine-3-carboxylate derivatives and biological evaluation as potential anticancer agents. Eur. J. Med. Chem. 2013, 69, 817–822. [Google Scholar] [CrossRef] [PubMed]
- El-Damasy, A.K.; Cho, N.C.; Pae, A.N.; Kim, E.E.; Keum, G. Novel 5-substituted-2-anilinoquinolines with 3-(morpholino or 4-methylpiperazin-1-yl)propoxy moiety as broad spectrum antiproliferative agents: Synthesis, cell based assays and kinase screening. Bioorg. Med. Chem. Lett. 2016, 26, 3307–3312. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W.; Seo, S.; Cho, K.H.; Alanazi, M.M.; Bang, E.K.; Keum, G.; El-Damasy, A.K. Discovery of New Quinoline-Based Diarylamides as Potent B-RAFV600E/C-RAF Kinase Inhibitors Endowed with Promising In Vitro Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 3216. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Gouda, M.A.; Baashen, M.; Alharbi, O.; Almalki, F.A.; Ranganatha, L.V. Piperazine Heterocycles as Potential Anticancer Agents: A Review. Pharm. Chem. J. 2022, 56, 29–37. [Google Scholar] [CrossRef]
- El-Damasy, A.K.; Cho, N.C.; Kang, S.B.; Pae, A.N.; Keum, G. ABL kinase inhibitory and antiproliferative activity of novel picolinamide based benzothiazoles. Bioorg. Med. Chem. Lett. 2015, 25, 2162–2168. [Google Scholar] [CrossRef]
- Richard, D.J.; Verheijen, J.C.; Curran, K.; Kaplan, J.; Toral-Barza, L.; Hollander, I.; Lucas, J.; Yu, K.; Zask, A. Incorporation of water-solubilizing groups in pyrazolopyrimidine mTOR inhibitors: Discovery of highly potent and selective analogs with improved human microsomal stability. Bioorg. Med. Chem. Lett. 2009, 19, 6830–6835. [Google Scholar] [CrossRef]
- Gao, H.; Marhefka, C.; Jacobs, M.D.; Cao, J.; Bandarage, U.K.; Green, J. ROCK inhibitors 2. Improving potency, selectivity and solubility through the application of rationally designed solubilizing groups. Bioorg. Med. Chem. Lett. 2018, 28, 2616–2621. [Google Scholar] [CrossRef]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.D.; McGown, A.T.; Lawrence, N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: Synthesis and biological evaluation of antivascular activity. Bioorg. Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, Q.; Jiang, Q.; Mottamal, M.; Zhang, Q.; Zhu, N.; Burow, M.E.; Worthylake, R.A.; Wang, G. Discovery of a series of thiazole derivatives as novel inhibitors of metastatic cancer cell migration and invasion. ACS Med. Chem. Lett. 2013, 4, 191–196. [Google Scholar] [CrossRef]
- DTP Human Tumor Cell Line Screen Process. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 4 May 2023).
- Lee, J.H.; El-Damasy, A.K.; Seo, S.H.; Gadhe, C.G.; Pae, A.N.; Jeong, N.; Hong, S.S.; Keum, G. Novel 5, 6-disubstituted pyrrolo [2,3-d] pyrimidine derivatives as broad spectrum antiproliferative agents: Synthesis, cell based assays, kinase profile and molecular docking study. Bioorg. Med. Chem. 2018, 26, 5596–5611. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://dtp.cancer.gov/organization/dscb/compoundSubmission/default.htm (accessed on 4 May 2023).
- Sabry, M.A.; Ewida, H.A.; Hassan, G.S.; Ghaly, M.A.; El-Subbagh, H.I. Synthesis, antitumor testing and molecular modeling study of some new 6-substituted amido, azo or thioureido-quinazolin-4 (3H)-ones. Bioorg. Chem. 2019, 88, 102923. [Google Scholar] [CrossRef] [PubMed]
- Grob, S. Molinspiration Cheminformatics Free Web Services. Available online: https://www.molinspiration.com (accessed on 4 May 2023).
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, 32–38. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717–42720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PreADMET, an Online ADMET Properties Predictor. Available online: https://preadmet.webse0072vice.bmdrc.org/ (accessed on 4 May 2023).





| Cancer Type | Cell Line | % Growth Inhibition | |||
|---|---|---|---|---|---|
| 4b (NSC Code 789748) | 4c (NSC Code 789749) | 4k (NSC Code 789663) | 4n (NSC Code 789664) | ||
| Mean GI% | 32.20 | 21.40 | 19.40 | 16.44 | |
| Leukemia | K-562 | 38.81 | 38.60 | 40.22 | 8.74 |
| MOLT-4 | 42.45 | 52.58 | 26.07 | 33.65 | |
| RPMI-8226 | 45.32 | NT | 42.85 | 33.53 | |
| SR | 61.31 | 22.65 | 34.42 | 23.02 | |
| Non-Small-Cell Lung Cancer | HOP-92 | 86.28 | NT | 64.64 | 47.14 |
| NCI-H522 | 38.29 | 60.42 | 54.63 | 45.23 | |
| Colon Cancer | HCT-116 | 46.35 | 39.35 | 13.70 | 23.40 |
| HCT-15 | 40.25 | 25.94 | 13.84 | 8.04 | |
| HT29 | 59.05 | 35.34 | 62.20 | 6.32 | |
| CNS Cancer | SF-295 | 73.52 | 15.35 | 2.09 | 15.19 |
| SNB-75 | 43.32 | 17.48 | 46.58 | 35.64 | |
| U251 | 41.82 | 12.14 | 20.14 | 22.93 | |
| Melanoma | LOX IMVI | 57.10 | 17.75 | 16.06 | 15.60 |
| SK-MEL-5 | 26.35 | 60.01 | 8.82 | 19.03 | |
| UACC-257 | 9.91 | 45.07 | 10.78 | 1.86 | |
| Renal Cancer | 786-0 | 36.71 | 0.36 | 45.28 | 33.67 |
| A498 | 72.38 | 24.94 | 15.91 | 11.18 | |
| Prostate Cancer | PC-3 | 38.70 | NT | 43.82 | 42.05 |
| Breast Cancer | MDA-MB-231 | 41.61 | 9.20 | 12.02 | 14.09 |
| HS 578T | 56.66 | −8.98 | 21.09 | 11.50 | |
| T-47D | 70.54 | 62.98 | 51.03 | 68.39 | |
| BT-549 | 49.20 | 13.39 | 38.24 | 43.30 | |
| Compound | % Growth Inhibition | |||||||
|---|---|---|---|---|---|---|---|---|
| MCF-7 | SK-BR-3 | HCT-116 | K562 | |||||
| 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | |
| 4a | 96.03 | 35.86 | 91.55 | 28.22 | 95.50 | 40.87 | 79.73 | 13.57 |
| 4b | 95.35 | 18.76 | 90.77 | 20.50 | 94.53 | 25.43 | 92.67 | 16.13 |
| 4c | 43.33 | 36.26 | 39.64 | 25.13 | 32.95 | 23.66 | 22.52 | 16.21 |
| 4d | 43.24 | 23.88 | 45.52 | 18.25 | 31.54 | 17.41 | 9.20 | 20.13 |
| 4e | 50.70 | 36.91 | 47.57 | 26.63 | 49.79 | 32.41 | 49.57 | 24.93 |
| 4f | 32.42 | 6.98 | 33.38 | 13.18 | 28.18 | 9.97 | 7.17 | 18.20 |
| 4g | 40.41 | 16.68 | 45.72 | 19.64 | 55.49 | 35.23 | 28.15 | 16.06 |
| 4h | 95.72 | 18.88 | 90.61 | 46.14 | 94.99 | 28.28 | 92.86 | 10.39 |
| 4i | 85.90 | 11.03 | 88.30 | 21.83 | 92.67 | 5.63 | 74.12 | 8.47 |
| 4j | 84.12 | 5.61 | 82.54 | 20.04 | 92.04 | 12.56 | 84.50 | 11.98 |
| 4k | 94.96 | 4.12 | 90.82 | 36.57 | 94.44 | 19.19 | 92.84 | 7.44 |
| 4l | 94.82 | 9.45 | 90.17 | 13.23 | 94.00 | 7.68 | 91.67 | 17.98 |
| 4m | 95.43 | 16.27 | 90.95 | 12.52 | 94.62 | 25.99 | 93.00 | 6.59 |
| 4n | 92.04 | 1.21 | 88.70 | 22.61 | 93.89 | 6.61 | 88.28 | 7.65 |
| Sorafenib | 96.06 | 40.45 | 93.12 | 48.87 | 97.32 | 48.41 | NT | NT |
| Compound | GI50 (μM) |
|---|---|
| 4a | 23.52 ± 2.77 |
| 4h | 26.38 ± 1.42 |
| Compound | Molinspiration Bioactivity Score | |||||
|---|---|---|---|---|---|---|
| GPCR Ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor | |
| 4a | 0.08 | −0.15 | 0.30 | −0.52 | −0.30 | 0.01 |
| 4b | 0.09 | −0.14 | 0.35 | −0.46 | −0.25 | 0.05 |
| 4h | 0.10 | −0.15 | 0.38 | −0.51 | −0.19 | 0.05 |
| Compound | List of Kinase Targets |
|---|---|
| 4a | INSR, ALK, IGF1R, CCND1 CDK4, PLK1, PLK4, CDK1, CDK4, FGFR4, PDK1 |
| 4b | TYRO3, CDK4, CCND1 CDK4, PLK4, MAPK1, IGF1R |
| 4h | IGF1R, CDK1, CDK4, ALK, AKT1, PLK1, PLK4, MET, TYRO3, INSR |
| Compound | Parameter | nVs(g) | |||||
|---|---|---|---|---|---|---|---|
| Log P(a) | TPSA(b) | MW(c) | nHBA(d) | nHBD(e) | nRB(f) | ||
| 4a | 4.06 | 113.11 | 470.59 | 7 | 1 | 8 | 0 |
| 4b | 3.77 | 133.34 | 486.59 | 8 | 2 | 9 | 0 |
| 4h | 4.32 | 125.14 | 499.63 | 8 | 2 | 10 | 0 |
| Compound | HIA% | Log S | CYP1A2 Inhibition | Carcinogenicity |
|---|---|---|---|---|
| 4a | 98.05 | −5.16 | Non-inhibitor | Non-carcinogenic |
| 4b | 95.68 | −4.52 | Non-inhibitor | Non-carcinogenic |
| 4h | 96.11 | −4.90 | Non-inhibitor | Non-carcinogenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Damasy, A.K.; Jin, H.; Sabry, M.A.; Kim, H.J.; Alanazi, M.M.; Seo, S.H.; Bang, E.-K.; Keum, G. Design and Synthesis of New 4-(3,4,5-Trimethoxyphenyl)Thiazole–Pyrimidine Derivatives as Potential Antiproliferative Agents. Medicina 2023, 59, 1076. https://doi.org/10.3390/medicina59061076
El-Damasy AK, Jin H, Sabry MA, Kim HJ, Alanazi MM, Seo SH, Bang E-K, Keum G. Design and Synthesis of New 4-(3,4,5-Trimethoxyphenyl)Thiazole–Pyrimidine Derivatives as Potential Antiproliferative Agents. Medicina. 2023; 59(6):1076. https://doi.org/10.3390/medicina59061076
Chicago/Turabian StyleEl-Damasy, Ashraf K., Heewon Jin, Mohamed A. Sabry, Hyun Ji Kim, Mohammed M. Alanazi, Seon Hee Seo, Eun-Kyoung Bang, and Gyochang Keum. 2023. "Design and Synthesis of New 4-(3,4,5-Trimethoxyphenyl)Thiazole–Pyrimidine Derivatives as Potential Antiproliferative Agents" Medicina 59, no. 6: 1076. https://doi.org/10.3390/medicina59061076