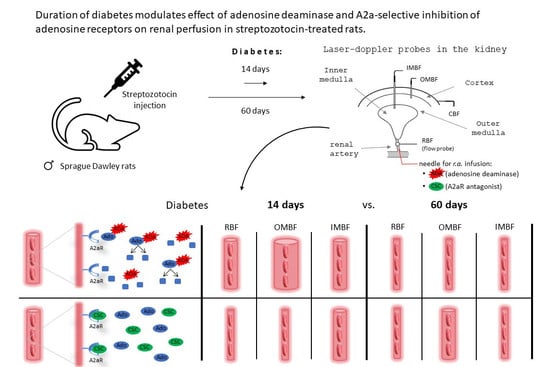
Nonselective and A2a-Selective Inhibition of Adenosine Receptors Modulates Renal Perfusion and Excretion Depending on the Duration of Streptozotocin-Induced Diabetes in Rats
Abstract
:1. Introduction
2. Results
2.1. Chronic Effects of Streptozotocin Pretreatment on Metabolic and Renal Excretion Parameters
2.2. Short- (14 Days) and Long-Term (60 Days) Effects of STZ-Induced Hyperglycaemia on Blood Pressure and Renal Haemodynamics and Excretion in Anaesthetised Rats
2.3. ADA Effects on MABP and Total and Regional Blood Circulation in Kidneys of NG and DM Rats
2.4. ADA Effects on Excretory Function in Kidneys of NG and DM Rats
2.5. CSC Effects on MABP, and Total and Regional Blood Circulation in Kidneys of NG and DM Rats
2.6. CSC Effects on Excretory Function in Kidneys of NG and DM Rats
2.7. ADA Effects on Tissue NO and H2O2 Signals in Kidneys of NG and DM Rats
2.8. CSC Effects on Tissue NO and H2O2 Signal in Kidneys of NG and DM Rats
3. Discussion
- (1)
- The present study compared the role of endogenous adenosine and its interaction with NO and H2O2 in controlling renal and systemic circulation and renal excretion between rats without diabetes and those with streptozotocin-induced diabetes.
- (2)
- The study was extended to compare these relationships between short- and long-term diabetic and age-matched normoglycaemic rats.
- (3)
- We postulated that in the kidney, the role of endogenous adenosine on circulation and excretion differs, depending on the actual duration of the experimental diabetes.
3.1. Chronic Observations
Duration of Hyperglycaemia and Renal Excretion
3.2. Blockade of Endogenous Ado and Renal Circulation
3.3. Endogenous Ado Blockade and Renal Excretion
3.4. Blockade of A2a Receptors and Renal Circulation
3.5. Blockade of A2a Receptors and Renal Excretion
3.6. Tissue NO and H2O2 and the Effects of Endogenous Ado System
3.7. Limitations of the Study and Future Research Targets
3.8. Concluding Remarks
3.8.1. The Duration of Diabetes Alters the Impact of P1R on Renal Perfusion
- −
- short-lasting diabetes (2 weeks, DM-14) can slightly modify the joint effect of all P1R on renal blood perfusion; however, this modification differs in the cortical and medullary regions. In the cortex, the vasoconstrictor effect prevails in normoglycaemic rats, but an inverse pattern is observed in hyperglycaemia. In general, the vasoconstrictor effect of P1R is most pronounced in the medulla of diabetic animals. However, in DM rats, the vasodilator impact of A2aR does not differ between the cortex and medulla.
- −
- long-lasting diabetes (8 weeks, DM-60) distinctly modifies the joint effect of all P1R on renal perfusion, but with no crucial difference between cortical and medullary circulation. In both regions, a constrictor effect (most probably A1R-dependent) prevails over dilatory action (most probably A2R dependent) in normoglycaemia, but this pattern is reversed in long-term diabetes.
3.8.2. The Duration of Diabetes Can Also Alter the Impact of P1R on Renal Excretion
- −
- short-lasting diabetes does not alter the net effect of all P1R on renal excretion, but can modify the impact of particular subtypes, such as A2aR.
- −
- long-lasting diabetes blunts the joint impact of all P1R on urine sodium excretion, and also its concentration; however, it does not alter A2aR contributions to the control of renal excretory functions.
3.8.3. The Duration of Diabetes Can Alter the Contribution of Individual or All P1R in Renal Tissue NO and H2O2 Availability
4. Materials and Methods
4.1. Animals
4.2. Induction of Diabetes
4.3. Chronic Studies
4.4. Acute Experiments
4.4.1. Surgical Preparations
4.4.2. Experimental Protocol
- normoglycaemic rats + ADA (NG + ADA)
- diabetic rats + ADA (DM + ADA)
- normoglycaemic rats + Ringer’s solution (NG + Rs)
- diabetic rats + Ringer’s solution (DM + Rs)
- normoglycaemic rats + CSC (NG + CSC)
- diabetic rats + CSC (DM + CSC)
- normoglycaemic rats + DMSO (NG + DMSO)
- diabetic rats + DMSO (DM + DMSO)
4.4.3. Analytical Procedures and Calculations
4.4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonioli, L.; Blandizzi, C.; Csóka, B.; Pacher, P.; Haskó, G. Adenosine signalling in diabetes mellitus—Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2015, 11, 228–241. [Google Scholar] [CrossRef]
- Peleli, M.; Carlstrom, M. Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol. Asp. Med. 2017, 55, 62–74. [Google Scholar] [CrossRef]
- Komers, R.; Anderson, S. Paradoxes of nitric oxide in the diabetic kidney. Am. J. Physiol. Physiol. 2003, 284, F1121–F1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palm, F.; Cederberg, J.; Hansell, P.; Liss, P.; Carlsson, P.-O. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 2003, 46, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative Stress as a Major Culprit in Kidney Disease in Diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, P.B.; Schnermann, J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am. J. Physiol. Physiol. 2003, 285, F590–F599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Ogawa, S.; Cowely, A.W.; Ito, S. Role of renal medullary oxidative and/or carbonyl stress in salt-sensitive hypertension and diabetes. Clin. Exp. Pharmacol. Physiol. 2012, 39, 125–131. [Google Scholar] [CrossRef]
- Palm, F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 997–1001. [Google Scholar] [CrossRef]
- Vitzthum, H.; Weiss, B.; Bachleitner, W.; Krämer, B.K.; Kurtz, A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004, 65, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G.; Novak, I. Purinergic signalling and diabetes. Purinergic Signal. 2013, 9, 307–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflueger, A.C.; Schenk, F.; Osswald, H. Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats. Am. J. Physiol. Physiol. 1995, 269, F529–F535. [Google Scholar] [CrossRef]
- Pawelczyk, T.; Grden, M.; Rzepko, R.; Sakowicz, M.; Szutowicz, A. Region-Specific Alterations of Adenosine Receptors Expression Level in Kidney of Diabetic Rat. Am. J. Pathol. 2005, 167, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Godinho, R.O.; Duarte, T.M.; Pacini, E.S.A.M. New perspectives in signaling mediated by receptors coupled to stimulatory G protein: The emerging significance of cAMP efflux and extracellular cAMP-adenosine pathway. Front. Pharmacol. 2015, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.S.; Huang, L.; Ye, H.; Duong, E.T.A.; Bolton, W.K.; Linden, J.; Okusa, M.D. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 290, F828–F837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhakar, S.S. Pathogenic role of nitric oxide alterations in diabetic nephropathy. Curr. Diabetes Rep. 2005, 5, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tran, S.; Sachetelli, S.; Filep, J.G.; Ingelfinger, J.R.; Chan JS, D. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007, 71, 912–923. [Google Scholar] [CrossRef] [Green Version]
- Erdely, A.; Freshour, G.; Maddox, D.A.; Olson, J.L.; Samsell, L.; Baylis, C. Renal disease in rats with Type 2 diabetes is associated with decreased renal nitric oxide production. Diabetologia 2004, 47, 1672–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumtaz, F.H.; Dashwood, M.R.; Khan, M.A.; Thompson, C.S.; Mikhailidis, D.P.; Morgan, R.J. Down-regulation of nitric oxide synthase in the diabetic rabbit kidney: Potential relevance to the early pathogenesis of diabetic nephropathy. Curr. Med. Res. Opin. 2004, 20, 1–6. [Google Scholar] [CrossRef]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic Nephropathy Is Associated with Oxidative Stress and Decreased Renal Nitric Oxide Production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [CrossRef] [Green Version]
- Kuczeriszka, M.; Sitek, J.D.; Walkowska, A.; Sadowski, J.; Dobrowolski, L. Interplay of the adenosine system and NO in control of renal haemodynamics and excretion: Comparison of normoglycaemic and streptozotocin diabetic rats. Nitric Oxide 2020, 104, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Patinha, D.; Afonso, J.; Sousa, T.; Morato, M.; Albino-Teixeira, A. Diabetes-induced increase of renal medullary hydrogen peroxide and urinary angiotensinogen is similar in normotensive and hypertensive rats. Life Sci. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Sousa, J.B.; Diniz, C. The Adenosinergic System as a Therapeutic Target in the Vasculature: New Ligands and Challenges. Molecules 2017, 22, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima, A.C.; Chaves, L.M.; Prestes, S.N.; Mânica, A.; Cardoso, A.M. The purinergic signalling and inflammation in the pathogenesis and progression of diabetes: Key factors and therapeutic targets. Inflamm. Res. 2022, 71, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Kuczeriszka, M.; Dobrowolski, L.; Walkowska, A.; Baranowska, I.; Sitek, J.D.; Kompanowska-Jezierska, E. Role of Ang1-7 in renal haemodynamics and excretion in streptozotocin diabetic rats. Clin. Exp. Pharmacol. Physiol. 2022, 49, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Kanarek, R.B. Dietary Self-Selection Patterns of Rats with Mild Diabetes. J. Nutr. 1985, 115, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Vanderweele, D.A. Insulin and satiety from feeding in pancreatic-normal and diabetic rats. Physiol. Behav. 1993, 54, 477–485. [Google Scholar] [CrossRef]
- Hidaka, S.; Yoshimatsu, H.; Kondou, S.; Oka, K.; Tsuruta, Y.; Sakino, H.; Itateyama, E.; Noguchi, H.; Himeno, K.; Okamoto, K.; et al. Hypoleptinemia, but not hypoinsulinemia, induces hyperphagia in streptozotocin-induced diabetic rats. J. Neurochem. 2001, 77, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Khamaisi, M.; Keynan, S.; Bursztyn, M.; Dahan, R.; Reinhartz, E.; Ovadia, H.; Raz, I. Role of Renal Nitric Oxide Synthase in Diabetic Kidney Disease during the Chronic Phase of Diabetes. Nephron Physiol. 2006, 102, 72–80. [Google Scholar] [CrossRef]
- Persson, P.; Friederich-Persson, M.; Fasching, A.; Hansell, P.; Inagi, R.; Palm, F. Adenosine A2a receptor stimulation prevents proteinuria in diabetic rats by promoting an anti-inflammatory phenotype without affecting oxidative stress. Acta Physiol. 2015, 214, 311–318. [Google Scholar] [CrossRef]
- Brands, M.W.; Bell, T.D.; Gibson, B. Nitric Oxide May Prevent Hypertension Early in Diabetes by Counteracting Renal Actions of Superoxide. Hypertension 2004, 43, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Vasilios, G.L. Hyponatremia in Diabetes Mellitus: Clues to Diagnosis and Treatment. J. Diabetes Metab. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Bankir, L.; Bardoux, P.; Ahloulay, M. Vasopressin and Diabetes mellitus. Nephron 2001, 87, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola, A.M.; Inscho, E.W.; Vari, R.C.; Navar, L.G. Influence of adenosine receptor blockade on renal function and renal autoregulation. J. Am. Soc. Nephrol. 1991, 2, 991–999. [Google Scholar] [CrossRef]
- Vallon, V.; Osswald, H. Adenosine receptors and the kidney. Adenosine Receptors in Health and Disease. In Adenosine Receptors in Health and Disease. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 443–470. [Google Scholar] [CrossRef]
- Yap, S.C.; Lee, H.T. Adenosine and protection from acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2012, 21, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spielman, W.S.; Arend, L.J. Adenosine receptors and signaling in the kidney. Hypertension 1991, 17, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Vallon, V.; Osswald, H. Dipyridamole prevents diabetes-induced alterations of kidney function in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994, 349, 217–222. [Google Scholar] [CrossRef]
- Patinha, D.; Afonso, J.; Sousa, T.; Morato, M.; Albino-Teixeira, A. Activation of adenosine receptors improves renal antioxidant status in diabetic Wistar but not SHR rats. Upsala J. Med. Sci. 2014, 119, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, L.; Kompanowska-Jezierska, E.; Walkowska, A.; Sadowski, J. Sodium intake determines the role of adenosine A2 receptors in control of renal medullary perfusion in the rat. Nephrol. Dial. Transplant. 2007, 22, 2805–2809. [Google Scholar] [CrossRef] [Green Version]
- Oyarzún, C.; Garrido, W.; Alarcón, S.; Yáñez, A.; Sobrevia, L.; Quezada, C.; Martín, R.S. Adenosine contribution to normal renal physiology and chronic kidney disease. Mol. Asp. Med. 2017, 55, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Eisenstein, A.; Patterson, S.; Ravid, K. The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases. J. Cell. Physiol. 2015, 230, 2891–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, A.; Skelton, M.M.; Zou, A.-P.; Cowley, A.W. Increased Renal Medullary H2O2 Leads to Hypertension. Hypertension 2003, 42, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-F.; Cowley, A.W.; Zou, A.-P. Increased H2O2counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R827–R833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassell, N.F.; Sprowell, J.A.; Boarini, D.J.; Olin, J.J. Effect of Dimethyl Sulfoxide on the Cerebral and Systemic Circulations of the Dog. Neurosurgery 1983, 12, 24–28. [Google Scholar] [CrossRef]
- Pitts, L.H.; Young, A.R.; McCulloch, J.; MacKenzie, E. Vasomotor effects of dimethyl sulfoxide on cat cerebral arteries in vitro and in vivo. Stroke 1986, 17, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkan, S.; Tuncok, Y.; Oransay, K.; Hocaoglu, N.; Buyukdeligoz, M. The role of adenosine receptors and endogenous adenosine in citalopram-induced cardiovascular toxicity. Indian J. Pharmacol. 2014, 46, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar] [CrossRef]
- Ba̧dzyńska, B.; Grzelec-Mojzesowicz, M.; Dobrowolski, L.; Sadowski, J. Differential effect of angiotensin II on blood circulation in the renal medulla and cortex of anaesthetised rats. J. Physiol. 2002, 538, 159–166. [Google Scholar] [CrossRef]
- Grzelec-Mojzesowicz, M.; Sadowski, J. Renal tissue NO and intrarenal haemodynamics during experimental vari-ations of NO content in anaesthetised rats. J. Physiol. Pharmacol. 2007, 58, 149. [Google Scholar]
- Zhang, X.; Broderick, M. Amperometric detection of nitric oxide. Mod. Asp. Immunobiol. 2000, 1, 160–165. [Google Scholar]







| Days After Injection | NG | DM | |||
|---|---|---|---|---|---|
| N | N | ||||
| MABP | 14 | 125 ± 3 | 19 | 129 ± 3 | 18 |
| (mmHg) | 60 | 134 ± 3 & | 17 | 124 ± 3 * | 19 |
| RBF | 14 | 4.8 ± 0.2 | 19 | 4.3 ± 0.4 | 18 |
| (mL/min/g kidney weight) | 60 | 4.8 ± 0.3 | 17 | 2.5 ± 0.2 *& | 19 |
| RVR | 14 | 27 ± 2 | 19 | 35 ± 4 | 18 |
| (mmHg min/mL) | 60 | 31 ± 4 | 17 | 53 ± 5 *& | 19 |
| V | 14 | 6.5 ± 0.8 | 17 | 11.1 ± 1.2 * | 21 |
| (µL/min/g kidney weight) | 60 | 5.2 ± 0.7 | 15 | 11.6 ± 1.5 * | 20 |
| Uosm | 14 | 858 ± 71 | 21 | 940 ± 49 | 21 |
| (mosmol/kg H2O) | 60 | 1019 ± 96 | 19 | 910 ± 39 | 20 |
| UosmV | 14 | 5.5 ± 0.5 | 19 | 9.8 ± 0.9 * | 21 |
| (µosmol/min/g kidney weight) | 60 | 4.8 ± 0.4 | 19 | 10.2 ± 1.2 * | 20 |
| UNaV | 14 | 0.6 ± 0.1 | 17 | 1.0 ± 0.2 | 19 |
| (µmol/min/g kidney weight) | 60 | 0.5 ± 0.07 | 18 | 1.0 ± 0.2 * | 18 |
| UKV | 14 | 1.1 ± 0.1 | 18 | 0.7 ± 0.1 * | 18 |
| (µmol/min/g kidney weight) | 60 | 0.7 ± 0.1 & | 14 | 0.8 ± 0.1 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitek, J.D.; Kuczeriszka, M.; Walkowska, A.; Kompanowska-Jezierska, E.; Dobrowolski, L. Nonselective and A2a-Selective Inhibition of Adenosine Receptors Modulates Renal Perfusion and Excretion Depending on the Duration of Streptozotocin-Induced Diabetes in Rats. Pharmaceuticals 2023, 16, 732. https://doi.org/10.3390/ph16050732
Sitek JD, Kuczeriszka M, Walkowska A, Kompanowska-Jezierska E, Dobrowolski L. Nonselective and A2a-Selective Inhibition of Adenosine Receptors Modulates Renal Perfusion and Excretion Depending on the Duration of Streptozotocin-Induced Diabetes in Rats. Pharmaceuticals. 2023; 16(5):732. https://doi.org/10.3390/ph16050732
Chicago/Turabian StyleSitek, Joanna Dorota, Marta Kuczeriszka, Agnieszka Walkowska, Elżbieta Kompanowska-Jezierska, and Leszek Dobrowolski. 2023. "Nonselective and A2a-Selective Inhibition of Adenosine Receptors Modulates Renal Perfusion and Excretion Depending on the Duration of Streptozotocin-Induced Diabetes in Rats" Pharmaceuticals 16, no. 5: 732. https://doi.org/10.3390/ph16050732