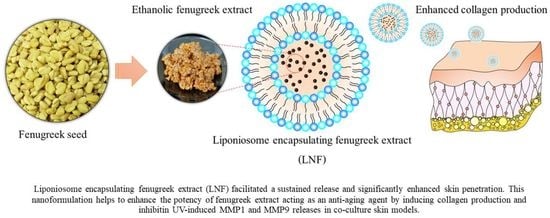
Ethanolic Fenugreek Extract: Its Molecular Mechanisms against Skin Aging and the Enhanced Functions by Nanoencapsulation
Abstract
:1. Introduction
2. Results
2.1. UHPLC Validation and Identification of Rutin in Fenugreek Extract
2.2. In Vitro Collagenase Inhibition and Collagen Production of Fenugreek Extract
2.3. Physicochemical Characterizations of Formulated LNF
2.4. Releasing Profile and Skin Penetration of Formulated LNF
2.5. Effect of LNF and Fenugreek Extract on Cell Viability and Collagen Production in Human Dermal Fibroblast Cells
2.6. Role of Formulated LNF on MMP1, MMP9, IL-6, and IL-8 Inhibition after UV Exposure
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Chemical Reagents
4.2. Extraction
4.3. UHPLC Validation and Identification of Rutin in Fenugreek Extract
4.4. Collagenase Assay
4.5. Cell Culture
4.6. Collagen Content and Picrosirius Red Staining
4.7. Cell Viability Assay
4.8. Liponiosomes Formulation
4.9. Percentages of Encapsulation Efficiency and Bioactive Loading
4.10. Franz Diffusion Cell
4.11. Porcine Skin Permeabilization
4.12. Differential Scanning Calorimeters (DSC) Characterization
4.13. Construction of Human Co-Cultured Skin Cells
4.14. Investigation of Cell Viability and MMPs Secretion after UV Exposure in Co-Cultured Skin Cells
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-Endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin Ageing: Natural Weapons and Strategies. Evid. Based Complement. Altern. Med. 2013, 10, 827248. [Google Scholar] [CrossRef] [Green Version]
- Madan, K.; Nanda, S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef]
- Yepes, A.; Ochoa-Bautista, D.; Murillo-Arango, W.; Quintero-Saumeth, J.; Bravo, K.; Osorio, E. Purple passion fruit seeds (Passiflora edulis f. edulis Sims) as a promising source of skin anti-aging agents: Enzymatic, antioxidant and multi-level computational studies. Arab. J. Chem. 2021, 14, 102905. [Google Scholar] [CrossRef]
- Nantarat, N.; Mueller, M.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Chansakaow, S.; Sirithunyalug, J.; Leelapornpisid, P. Sesaminol diglucoside isolated from black sesame seed cake and its antioxidant, anti-collagenase and anti-hyaluronidase activities. Food Biosci. 2020, 36, 100628. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Oliveira, A.L.; Pedrosa, S.S.; Pintado, M.; Madureira, A.R. Potential of sugarcane extracts as cosmetic and skincare ingredients. Ind. Crops Prod. 2021, 169, 113625. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Application of Hibiscus cannabinus L. (kenaf) leaves extract as skin whitening and anti-aging agents in natural cosmetic prototype. Ind. Crops Prod. 2021, 167, 113491. [Google Scholar] [CrossRef]
- Song, J.; Zhou, Y.-Z.; Pang, Y.-Y.; Gao, L.; Du, G.-H.; Qin, X.-M. The anti-aging effect of Scutellaria baicalensis Georgi flowers extract by regulating the glutamine-glutamate metabolic pathway in d-galactose induced aging rats. Exp. Gerontol. 2020, 134, 110843. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, B.; Fendri, I.; Dahech, I.; Belghuith, K.; Kammoun, R.; Mejdoub, H. Purification and characterization of a maltogenic amylase from Fenugreek (Trigonella foenum graecum) seeds using the Box Benkhen Design (BBD). Ind. Crops Prod. 2013, 43, 334–339. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Ramamurty, N.; Gunasekaran, P.; Varalakshmi, E.; Anuradha, C.V. Fenugreek (Trigonella foenum graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006, 41, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neelakantan, N.; Narayanan, M.; de Souza, R.J.; van Dam, R.M. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr. J. 2014, 13, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanger, M.I.; Bukhari, S.B.; Memon, S. Antioxidative Activity of Extracts from a Fenugreek Seeds (Trigonella foenum-graecum). Pak. J. Anal. Environ. Chem. 2008, 9, 6. [Google Scholar]
- Rao, P.U.; Sesikeran, B.; Rao, P.S.; Naidu, A.N.; Rao, V.V.; Ramachandran, E.P. Short term nutritional and safety evaluation of fenugreek. Nutr. Res. 1996, 16, 1495–1505. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Identification and quantification of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) germinated seeds by LC–DAD–ESI/MS analysis. J. Food Compos. Anal. 2014, 35, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Madhava Naidu, M.; Shyamala, B.N.; Pura Naik, J.; Sulochanamma, G.; Srinivas, P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. LWT-Food Sci. Technol. 2011, 44, 451–456. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Shabbeer, S.; Sobolewski, M.; Anchoori, R.K.; Kachhap, S.; Hidalgo, M.; Jimeno, A.; Davidson, N.E.; Carducci, M.; Khan, S.R. Fenugreek: A naturally occurring edible spice as an anticancer agent. Cancer Biol. Ther. 2009, 8, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Sulieman, A.M.E.; Ahmed, H.E.; Abdelrahim, A.M. The Chemical Composition of Fenugreek (Trigonella foenum graceum L) and the Antimicrobial Properties of its Seed Oil. Gezira J. Eng. Appl. Sci. 2008, 3, 52–71. [Google Scholar]
- Sindhu, G.; Ratheesh, M.; Shyni, G.L.; Nambisan, B.; Helen, A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012, 12, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.; Meinke, M.; Keck, C.; Müller, R. Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Cervantes-Laurean, D.; Schramm, D.D.; Jacobson, E.L.; Halaweish, I.; Bruckner, G.G.; Boissonneault, G.A. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J. Nutr. Biochem. 2006, 17, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Vetrova, O.Y.; Kokorina, K.A.; Bazhaeva, Z.B.; Mel’nik, Y.V.; Petrova, Y.Y. Sorption-Catalytic Determination of Rutin, Lysine, and Collagen in Pharmaceuticals and Cosmetics. Pharm. Chem. J. 2015, 48, 753–758. [Google Scholar] [CrossRef]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mater. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef]
- Reza Mozafari, M.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef]
- Naderinezhad, S.; Amoabediny, G.; Haghiralsadat, F. Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible pH-sensitive lipid-based nano-carriers for multidrug-resistant cancers. RSC Adv. 2017, 7, 30008–30019. [Google Scholar] [CrossRef] [Green Version]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J. Drug Deliv. 2011, 2011, 418316. [Google Scholar] [CrossRef] [Green Version]
- AOAC. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Ankilam, E.; Heinze, P.; Kay, S.; Van den Eede, G.; Popping, B. Validation studies and proficiency testing. J. AOAC Int. 2002, 85, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.; Fisher, G. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Stipcevic, T.; Piljac, J.; Berghe, D.V. Effect of Different Flavonoids on Collagen Synthesis in Human Fibroblasts. Plant Foods Hum. Nutr. 2006, 61, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [Green Version]
- Król-Kogus, B.; Głód, D.; Krauze-Baranowska, M.; Matławska, I. Application of one- and two-dimensional high-performance liquid chromatography methodologies for the analysis of C-glycosylflavones from fenugreek seeds. J. Chromatogr. A 2014, 1367, 48–56. [Google Scholar] [CrossRef]
- Omezzine, F.; Bouaziz, M.; Daami-Remadi, M.; Simmonds, M.S.J.; Haouala, R. Chemical composition and antifungal activity of Trigonella foenum-graecum L. varied with plant ploidy level and developmental stage. Arab. J. Chem. 2017, 10, S3622–S3631. [Google Scholar] [CrossRef] [Green Version]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dhanabal, S.P. Development and optimization of bioanalytical parameters for the standardization of Trigonella foenum-graecum. J. Acute Dis. 2013, 2, 137–139. [Google Scholar] [CrossRef] [Green Version]
- Shailajan, S.; Menon, S.; Singh, A.; Mhatre, M.; Sayed, N. A validated RP-HPLC method for quantitation of trigonelline from herbal formulations containing Trigonella foenum-graecum (L.) seeds. Pharm. Methods 2011, 2, 157–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 200. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the Skin; JAAM: Tokyo, Japan, 2009; Volume 6, pp. 46–59. [Google Scholar]
- Raschke, C.; Elsner, P. Skin Aging: A Brief Summary of Characteristic Changes. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 37–43. [Google Scholar]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, X.; Wang, B.; Zhang, G.; Liu, M.; Geng, S.; Sun, L.; An, J.; Zhang, Z.; Zhang, H. A soft anti-virulence liposome realizing the explosive release of antibiotics at an infectious site to improve antimicrobial therapy. J. Mater. Chem. B 2021, 9, 147–158. [Google Scholar] [CrossRef]
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Investig. Dermatol. 1993, 100, S35–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Nothnagel, L.; Wacker, M.G. How to measure release from nanosized carriers? Eur. J. Pharm. Sci. 2018, 120, 199–211. [Google Scholar] [CrossRef]
- Yusuf, M.; Sharma, V.; Pathak, K. Nanovesicles for transdermal delivery of felodipine: Development, characterization, and pharmacokinetics. Int. J. Pharm. Investig. 2014, 4, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Parameters | Results |
|---|---|
| Linear range | |
| Equation | Y = 3308.80x − 15,055.67 |
| Coefficient of determination (r2) | 0.9998 |
| Accuracy (% Recovery) | |
| 100, 200, 400 µg/mL | 104.85, 106.16, 102.85 |
| Precision (% RSD * of % Recovery) | |
| Intra-day: 100, 200, 400 µg/mL Inter-day: 100, 200, 400 µg/mL | 0.99, 0.57, 0.34 1.00, 0.56, 0.24 |
| Sensitivity | |
| Limit of Detection: LOD (µg/mL) Limit of Quantitation LOQ (µg/mL) | 5.17 15.67 |
| Chemicals | % |
|---|---|
| Part A: oil phase | |
| Cholesterol | 5 |
| Propylene glycol | 10 |
| Sorbitan oleate | 15 |
| Phospholipid: soybean lecithin | 10 |
| Part B: water phase | |
| Fenugreek extract | 10 |
| Propylene glycol | 10 |
| DI water | 30 |
| Part C: Edge activator: Tocopherol acetate | 8 |
| Part D: Preservatives | 2 |
| Parameters | Initial |
|---|---|
| Size (nm) | 174.7 ± 49.2 |
| Polydispersity index (PdI) | 0.26 ± 0.04 |
| ζ Potential (mV) | −26.0 ± 1.2 |
| pH | 6.38 ± 0.05 |
| Viscosity (cP) | 49,930 ± 46 |
| % Encapsulation efficiency | 46.6 ± 7.4 |
| % Drug load | 33.5 ± 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eaknai, W.; Bunwatcharaphansakun, P.; Phungbun, C.; Jantimaporn, A.; Chaisri, S.; Boonrungsiman, S.; Nimmannit, U.; Khongkow, M. Ethanolic Fenugreek Extract: Its Molecular Mechanisms against Skin Aging and the Enhanced Functions by Nanoencapsulation. Pharmaceuticals 2022, 15, 254. https://doi.org/10.3390/ph15020254
Eaknai W, Bunwatcharaphansakun P, Phungbun C, Jantimaporn A, Chaisri S, Boonrungsiman S, Nimmannit U, Khongkow M. Ethanolic Fenugreek Extract: Its Molecular Mechanisms against Skin Aging and the Enhanced Functions by Nanoencapsulation. Pharmaceuticals. 2022; 15(2):254. https://doi.org/10.3390/ph15020254
Chicago/Turabian StyleEaknai, Waleewan, Phichaporn Bunwatcharaphansakun, Chutikorn Phungbun, Angkana Jantimaporn, Sasikan Chaisri, Suwimon Boonrungsiman, Ubonthip Nimmannit, and Mattaka Khongkow. 2022. "Ethanolic Fenugreek Extract: Its Molecular Mechanisms against Skin Aging and the Enhanced Functions by Nanoencapsulation" Pharmaceuticals 15, no. 2: 254. https://doi.org/10.3390/ph15020254