Antiparasitic Effect of Stilbene and Terphenyl Compounds against Trypanosoma cruzi Parasites
Abstract
:1. Introduction
2. Results
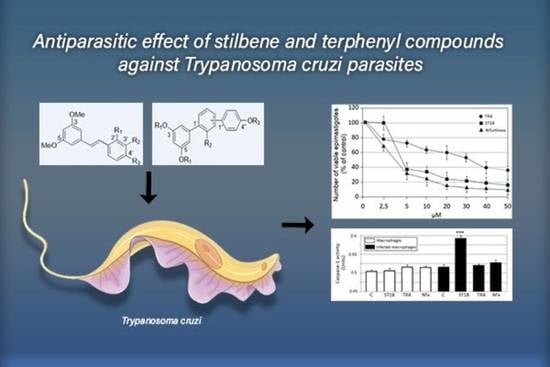
2.1. Anti-Trypanosoma cruzi Activity
2.2. Mammalian Cell Cytotoxicity and SI
2.3. Cell Cycle
2.4. Flow Cytometry Analysis of Physical Parameters (Cell Size and Granularity)
2.5. Annexin V and MDC Labeling
2.6. Caspase-1
3. Discussion
4. Materials and Methods
4.1. Parasite Cultures
4.2. Compound and Sample Preparation
4.3. Epimastigote Viability Assay
4.4. Effects of Compounds in Intracellular Amastigotes
4.5. Mammalian Cell Cytotoxicity
4.6. Cell Cycle Analysis by Flow Cytometry
4.7. Cell Volume Determination
4.8. Determination of Apoptosis by Annexin V
4.9. Monodansylcadaverine Labelling
4.10. Caspase-1 Detection
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoemaker, E.A.; Dale, K.; Cohn, D.A.; Kelly, M.P.; Zoerhoff, K.L.; Batcho, W.E.; Bougouma, C.; Nko’Ayissi, G.B.; Meite, A.; Marfo, B.; et al. Gender and Neglected Tropical Disease Front-Line Workers: Data from 16 Countries. PLoS ONE 2019, 14, e0224925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, N.F.; Gonzalez, M.S.; Gomes, J.E.; de Souza, W.; Garcia, E.S.; Azambuja, P.; Nohara, L.L.; Almeida, I.C.; Zingales, B.; Colli, W. Trypanosoma cruzi: Involvement of glycoinositolphospholipids in the attachment to the luminal midgut surface of Rhodnius prolixus. Exp. Parasitol. 2007, 116, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Paroli, A.F.; Gonzalez, P.V.; Díaz-Luján, C.; Onofrio, L.I.; Arocena, A.; Cano, R.C.; Carrera-Silva, E.A.; Gea, S. NLRP3 Inflammasome and Caspase-1/11 Pathway Orchestrate Different Outcomes in the Host Protection Against Trypanosoma cruzi Acute Infection. Front. Immunol. 2018, 9, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbina, J.A.; Docampo, R. Specific Chemotherapy of Chagas Disease: Controversies and Advances. Trends Parasitol. 2003, 19, 495–501. [Google Scholar] [CrossRef]
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic Side Effects of Drugs Used to Treat Chagas’ Disease (American Trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.R.; Conserva, G.A.A.; Varela, M.T.; Costa-Silva, T.A.; Thevenard, F.; Ponci, V.; Fortuna, A.; Falcão, A.C.; Tempone, A.G.; Fernandes, J.P.S.; et al. Improving the Drug-Likeness of Inspiring Natural Products—Evaluation of the Antiparasitic Activity against Trypanosoma Cruzi through Semi-Synthetic and Simplified Analogues of Licarin A. Sci. Rep. 2020, 10, 5467. [Google Scholar] [CrossRef]
- Pizzirani, D.; Roberti, M.; Cavalli, A.; Grimaudo, S.; Di Cristina, A.; Pipitone, R.M.; Gebbia, N.; Tolomeo, M.; Recanatini, M. Antiproliferative Agents That Interfere with the Cell Cycle at the G1→S Transition: Further Development and Characterization of a Small Library of Stilbene-Derived Compounds. ChemMedChem 2008, 3, 345–355. [Google Scholar] [CrossRef]
- Roberti, M.; Pizzirani, D.; Simoni, D.; Rondanin, R.; Baruchello, R.; Bonora, C.; Buscemi, F.; Grimaudo, S.; Tolomeo, M. Synthesis and Biological Evaluation of Resveratrol and Analogues as Apoptosis-Inducing Agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef]
- Roberti, M. Identification of a Terphenyl Derivative That Blocks the Cell Cycle in the G0−G1 Phase and Induces Differentiation in Leukemia Cells. J. Med. Chem. 2006, 49, 3012–3018. Available online: https://pubs.acs.org/doi/pdf/10.1021/jm060253o (accessed on 2 February 2021). [CrossRef]
- Tolomeo, M.; Roberti, M.; Scapozza, L.; Tarantelli, C.; Giacomini, E.; Titone, L.; Saporito, L.; Di Carlo, P.; Colomba, C. TTAS a New Stilbene Derivative That Induces Apoptosis in Leishmania Infantum. Exp. Parasitol. 2013, 133, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, G.; Bruno, F.; Vitale, F.; Roberti, M.; Colomba, C.; Giacomini, E.; Guidotti, L.; Cascio, A.; Tolomeo, M. In Vitro Antileishmanial Activity of Trans-Stilbene and Terphenyl Compounds. Exp. Parasitol. 2016, 166, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, F.; Castelli, G.; Vitale, F.; Giacomini, E.; Roberti, M.; Colomba, C.; Cascio, A.; Tolomeo, M. Effects of Trans-Stilbene and Terphenyl Compounds on Different Strains of Leishmania and on Cytokines Production from Infected Macrophages. Exp. Parasitol. 2018, 184, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ko, H.; Park, J.E.; Jung, S.; Lee, S.K.; Chun, Y.-J. Design, Synthesis, and Discovery of Novel Trans-Stilbene Analogues as Potent and Selective Human Cytochrome P450 1B1 Inhibitors. J. Med. Chem. 2002, 45, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Paredes, R.; Sosa, M.A.; Galanti, N. Natural Programmed Cell Death in T. Cruzi Epimastigotes Maintained in Axenic Cultures. J. Cell. Biochem. 2008, 105, 688–698. Available online: https://pubmed.ncbi.nlm.nih.gov/18668509/ (accessed on 15 February 2021). [CrossRef] [PubMed]
- Biederbick, A.; Kern, H.F.; Elsässer, H.P. Monodansylcadaverine (MDC) Is a Specific In Vivo Marker for Autophagic Vacuoles. Eur. J. Cell Biol. 1995, 66, 3–14. [Google Scholar]
- Lin, H.-S.; Ho, P.C. Preclinical Pharmacokinetic Evaluation of Resveratrol Trimethyl Ether in Sprague-Dawley Rats: The Impacts of Aqueous Solubility, Dose Escalation, Food and Repeated Dosing on Oral Bioavailability. J. Pharm. Sci. 2011, 100, 4491–4500. Available online: https://pubmed.ncbi.nlm.nih.gov/21520090/ (accessed on 15 February 2021). [CrossRef] [PubMed]
- Wang, T.Y.; Schoene, N.W.; Young, S.; Kim, Y.S.; Mizuno, C.S.; Rimando, A.M. Differential Effects of Resveratrol and Its Naturally Occurring Methylether Analogs on Cell Cycle and Apoptosis in Human Androgen-Responsive LNCaP Cancer Cells. Mol. Nutr. Food Res. 2010, 54, 335–344. Available online: https://pubmed.ncbi.nlm.nih.gov/20077416/ (accessed on 15 February 2021). [CrossRef]
- Weng, C.-J.; Yang, Y.-T.; Ho, C.-T.; Yen, G.-C. Mechanisms of Apoptotic Effects Induced by Resveratrol, Dibenzoylmethane, and Their Analogues on Human Lung Carcinoma Cells. J. Agric. Food Chem. 2009, 57, 5235–5243. Available online: https://pubmed.ncbi.nlm.nih.gov/19441815/ (accessed on 15 February 2021). [CrossRef]
- Yang, Y.-T.; Weng, C.-J.; Ho, C.-T.; Yen, G.-C. Resveratrol Analog-3,5,4’-Trimethoxy-Trans-Stilbene Inhibits Invasion of Human Lung Adenocarcinoma Cells by Suppressing the MAPK Pathway and Decreasing Matrix Metalloproteinase-2 Expression. Mol. Nutr. Food Res. 2009, 53, 407–416. Available online: https://pubmed.ncbi.nlm.nih.gov/19072741/ (accessed on 15 February 2021). [CrossRef]
- Bader, Y.; Madlener, S.; Strasser, S.; Maier, S.; Saiko, P.; Stark, N.; Popescu, R.; Huber, D.; Gollinger, M.; Erker, T.; et al. Stilbene Analogues Affect Cell Cycle Progression and Apoptosis Independently of Each Other in an MCF-7 Array of Clones with Distinct Genetic and Chemoresistant Backgrounds. Oncol. Rep. 2008, 19, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Liu, B.; Xiong, H.; Wu, F.; Hu, C.; Liu, P. Trans-3,5,4′-trimethoxystilbene Reduced Gefitinib Resistance in NSCLCs via Suppressing MAPK/Akt/Bcl-2 Pathway by Upregulation of MiR-345 and MiR-498. J. Cell. Mol. Med. 2019, 23, 2431–2441. [Google Scholar] [CrossRef] [Green Version]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-Resveratrol and Piceatannol Administered Orally Suppress and Inhibit Tumor Formation and Growth in Prostate Cancer Xenografts. Prostate 2013, 73, 1135–1146. Available online: https://pubmed.ncbi.nlm.nih.gov/23657951/ (accessed on 15 February 2021). [CrossRef]
- Rivera, H.; Shibayama, M.; Tsutsumi, V.; Perez-Alvarez, V.; Muriel, P. Resveratrol and Trimethylated Resveratrol Protect from Acute Liver Damage Induced by CCl4 in the Rat. J. Appl. Toxicol. 2008, 28, 147–155. [Google Scholar] [CrossRef]
- Liu, B.; Luo, X.-L.; Yang, Z.-B.; Zhang, J.-J.; Li, T.-B.; Zhang, X.-J.; Ma, Q.-L.; Zhang, G.-G.; Hu, C.-P.; Peng, J. Inhibition of NOX/VPO1 Pathway and Inflammatory Reaction by Trimethoxystilbene in Prevention of Cardiovascular Remodeling in Hypoxia-Induced Pulmonary Hypertensive Rats. J. Cardiovasc. Pharmacol. 2014, 63, 567–576. Available online: https://pubmed.ncbi.nlm.nih.gov/24492474/ (accessed on 15 February 2021). [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and Safety Profile of Trans-Resveratrol in a Rising Multiple-Dose Study in Healthy Volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 Mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharm. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, D.; Roberti, M.; Invidiata, F.P.; Aiello, E.; Aiello, S.; Marchetti, P.; Baruchello, R.; Eleopra, M.; Di Cristina, A.; Grimaudo, S.; et al. Stilbene-Based Anticancer Agents: Resveratrol Analogues Active toward HL60 Leukemic Cells with a Non-Specific Phase Mechanism. Bioorg. Med. Chem. Lett. 2006, 16, 3245–3248. Available online: https://pubmed.ncbi.nlm.nih.gov/16580204/ (accessed on 15 February 2021). [CrossRef]
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Corrado Tringali, C. Antiproliferative Activity of Methylated Analogues of E- and Z-Resveratrol. Z. Naturforsch. C 2007, 62, 189–195. Available online: https://pubmed.ncbi.nlm.nih.gov/17542483/ (accessed on 15 February 2021). [CrossRef] [PubMed]
- Pan, M.-H.; Gao, J.-H.; Lai, C.-S.; Wang, Y.-J.; Chen, W.-M.; Lo, C.-Y.; Wang, M.; Dushenkov, S.; Ho, C.-T. Antitumor Activity of 3,5,4′-Trimethoxystilbene in COLO 205 Cells and Xenografts in SCID Mice. Mol. Carcinog. 2008, 47, 184–196. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, J.; Liu, S.-Y.; Wang, C.-J.; Xiang, D.-X.; Xiong, X.-M.; Hu, C.-P.; Li, Y.-J. Inhibitory Effect of Resveratrol Derivative BTM-0512 on High Glucose-Induced Cell Senescence Involves Dimethylaminohydrolase/Asymmetric Dimethylarginine Pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-H.; Alex, D.; Huang, H.-Q.; Wang, N.; Yu, N.; Wang, Y.-T.; Leung, G.P.H.; Lee, S.M.Y. Inhibition of TNF-α-Mediated Endothelial Cell-Monocyte Cell Adhesion and Adhesion Molecules Expression by the Resveratrol Derivative, Trans-3,5,4’-Trimethoxystilbene. Phytother. Res. 2011, 25, 451–457. Available online: https://pubmed.ncbi.nlm.nih.gov/20740479/ (accessed on 15 February 2021). [CrossRef] [PubMed]
- Meng, X.-L.; Yang, J.-Y.; Chen, G.-L.; Wang, L.-H.; Zhang, L.-J.; Wang, S.; Li, L.; Wu, C.-F. Effects of Resveratrol and Its Derivatives on Lipopolysaccharide-Induced Microglial Activation and Their Structure-Activity Relationships. Chem. Biol. Interact. 2008, 174, 51–59. Available online: https://pubmed.ncbi.nlm.nih.gov/18513711/ (accessed on 15 February 2021). [CrossRef]
- Cho, D.I.; Koo, N.Y.; Chung, W.J.; Kim, T.S.; Ryu, S.Y.; Im, S.Y.; Kim, K.M. Effects of resveratrol-related hydroxystilben es on the nitric oxide production in macrophage cells: Structural requirements and mechanism of action. Life Sci. 2002, 71, 2071–2082. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Role of Dietary Polyphenols in the Management of Peptic Ulcer. World J. Gastroenterol. 2015, 21, 6499–6517. [Google Scholar] [CrossRef]
- Sholler, G.L.S.; Brard, L.; Straub, J.A.; Dorf, L.; Illyene, S.; Koto, K.; Kalkunte, S.; Bosenberg, M.; Ashikaga, T.; Nishi, R. Nifurtimox Induces Apoptosis of Neuroblastoma Cells in Vitro and in Vivo. J. Pediatr. Hematol. Oncol. 2009, 31, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Zhang, L.; Scorsone, K.A.; Woodfield, S.E.; Zage, P.E. Nifurtimox Is Effective Against Neural Tumor Cells and Is Synergistic with Buthionine Sulfoximine. Sci. Rep. 2016, 6, 27458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koto, K.S.; Lescault, P.; Brard, L.; Kim, K.; Singh, R.K.; Bond, J.; Illenye, S.; Slavik, M.A.; Ashikaga, T.; Saulnier Sholler, G.L. Antitumor Activity of Nifurtimox Is Enhanced with Tetrathiomolybdate in Medulloblastoma. Int. J. Oncol. 2011, 38, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Goldshmidt, H.; Matas, D.; Kabi, A.; Carmi, S.; Hope, R.; Michaeli, S. Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in Trypanosoma Brucei. PLoS Pathog. 2010, 6, e1000731. [Google Scholar] [CrossRef] [Green Version]
- Uzcátegui, N.L.; Carmona-Gutiérrez, D.; Denninger, V.; Schoenfeld, C.; Lang, F.; Figarella, K.; Duszenko, M. Antiproliferative Effect of Dihydroxyacetone on Trypanosoma Brucei Bloodstream Forms: Cell Cycle Progression, Subcellular Alterations, and Cell Death. Antimicrob. Agents Chemother. 2007, 51, 3960–3968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, M.; Anderson, P.; Garcia-Salcedo, J.A.; Caro, M.; Gonzalez-Rey, E. Neuropeptides Kill African Trypanosomes by Targeting Intracellular Compartments and Inducing Autophagic-like Cell Death. Cell Death Differ. 2009, 16, 406–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.T.; Yeoh, C.Y.; Zainuddin, Z.; Ilham Adenan, M. (+)-Spectaline and Iso-6-Spectaline Induce a Possible Cross-Talk between Autophagy and Apoptosis in Trypanosoma Brucei Rhodesiense. Trop. Med. Infect. Dis. 2019, 4, 98. [Google Scholar] [CrossRef] [Green Version]
- Ojha, R.; Ishaq, M.; Singh, S.K. Caspase-Mediated Crosstalk between Autophagy and Apoptosis: Mutual Adjustment or Matter of Dominance. J. Cancer Res. 2015, 11, 514–524. [Google Scholar] [CrossRef]
- Acharya, B.R.; Bhattacharyya, S.; Choudhury, D.; Chakrabarti, G. The Microtubule Depolymerizing Agent Naphthazarin Induces Both Apoptosis and Autophagy in A549 Lung Cancer Cells. Apoptosis 2011, 16, 924–939. [Google Scholar] [CrossRef]
- Reunanen, H.; Marttinen, M.; Hirsimäki, P. Effects of Griseofulvin and Nocodazole on the Accumulation of Autophagic Vacuoles in Ehrlich Ascites Tumor Cells. Exp. Mol. Pathol. 1988, 48, 97–102. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Hsu, Y.-L.; Cho, C.-Y. Plumbagin Induces G2-M Arrest and Autophagy by Inhibiting the AKT/Mammalian Target of Rapamycin Pathway in Breast Cancer Cells. Mol. Cancer Ther. 2006, 5, 3209–3221. [Google Scholar] [CrossRef] [Green Version]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The Role of Autophagy in Cancer Development and Response to Therapy. Nat. Rev. Cancer 2005, 5, 726–734. [Google Scholar] [CrossRef]
- Yu, J.; Nagasu, H.; Murakami, T.; Hoang, H.; Broderick, L.; Hoffman, H.M.; Horng, T. Inflammasome Activation Leads to Caspase-1-Dependent Mitochondrial Damage and Block of Mitophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 15514–15519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locksley, R.M.; Klebanoff, S.J. Oxygen-dependent Microbicidal Systems of Phagocytes and Host Defense against Intracellular Protozoa. J. Cell. Biochem. 1983, 22, 173–185. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jcb.240220306 (accessed on 2 February 2021). [CrossRef] [PubMed]
- Nathan, C.; Nogueira, N.; Juangbhanich, C.; Ellis, J.; Cohn, Z. Activation of Macrophages in Vivo and in Vitro. Correlation between Hydrogen Peroxide Release and Killing of Trypanosoma Cruzi. J. Exp. Med. 1979, 149, 1056–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, G.; Galante, A.; Lo Verde, V.; Migliazzo, A.; Reale, S.; Lupo, T.; Piazza, M.; Vitale, F.; Bruno, F. Evaluation of Two Modified Culture Media for Leishmania Infantum Cultivation versus Different Culture Media. J. Parasitol. 2014, 100, 228–230. [Google Scholar] [CrossRef] [PubMed]







| Compound | Structure | IC50 1 (µM) ± SE 2 |
|---|---|---|
| ST18 |  | 4.6 ± 0.4 |
| 1 |  | 34 ± 5.2 |
| 2 |  | >50 |
| 3 |  | >50 |
| 4 |  | >50 |
| 5 |  | 38 ± 5 |
| 6 |  | >50 |
| 7 |  | >50 |
| 8 |  | 48 ± 6.8 |
| 9 |  | 33 ± 4.9 |
| 10 |  | >50 |
| TR4 |  | 30 ± 4.3 |
| 11 |  | >50 |
| 12 |  | 34 ± 5.8 |
| 13 |  | 46 ± 6.3 |
| 14 |  | >50 |
| 15 |  | >50 |
| Nifurtimox |  | ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, F.; Castelli, G.; Vitale, F.; Catanzaro, S.; Badaco, V.V.; Roberti, M.; Colomba, C.; Cascio, A.; Tolomeo, M. Antiparasitic Effect of Stilbene and Terphenyl Compounds against Trypanosoma cruzi Parasites. Pharmaceuticals 2021, 14, 1199. https://doi.org/10.3390/ph14111199
Bruno F, Castelli G, Vitale F, Catanzaro S, Badaco VV, Roberti M, Colomba C, Cascio A, Tolomeo M. Antiparasitic Effect of Stilbene and Terphenyl Compounds against Trypanosoma cruzi Parasites. Pharmaceuticals. 2021; 14(11):1199. https://doi.org/10.3390/ph14111199
Chicago/Turabian StyleBruno, Federica, Germano Castelli, Fabrizio Vitale, Simone Catanzaro, Valeria Vitale Badaco, Marinella Roberti, Claudia Colomba, Antonio Cascio, and Manlio Tolomeo. 2021. "Antiparasitic Effect of Stilbene and Terphenyl Compounds against Trypanosoma cruzi Parasites" Pharmaceuticals 14, no. 11: 1199. https://doi.org/10.3390/ph14111199