Simple Binding and Dissociation of a Sialoglycoprotein Using Boronic Acid-Modified Functional Interfaces on Microparticles
Abstract
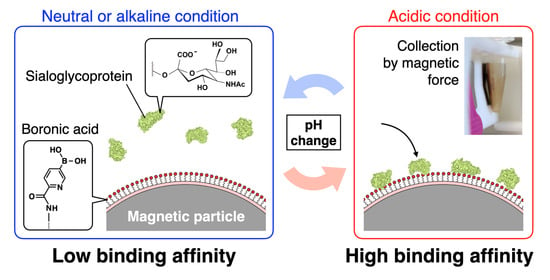
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Synthesis of 5-BPA–Terminated PEG
2.3. Preparation of the BMPs
2.4. Fetuin Collection Using the BMPs
2.5. Repeatability of Binding and Dissociation between Fetuin and the BMPs
2.6. Condensation of Fetuin Using BMPs
3. Results and Discussion
3.1. Preparation of Boronic Acid-Terminated PEG
3.2. Preparation of BMPs and Fetuin Capture Efficiency
3.3. Repeat Evaluation of Fetuin Capture and Release from BMPs
3.4. Effect of the Fetuin Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Simpson, A.B.; D’Costa, E.; Bunn, F.S.; van Leeuwen, S.S. Sialic acid, the secret gift for the brain. Crit. Rev. Food Sci. Nutr. 2023, 63, 9875–9894. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswaran, G.; Salk, P. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science 1981, 212, 1514–1516. [Google Scholar] [CrossRef] [PubMed]
- Kijima-Suda, I.; Miyamoto, Y.; Toyoshima, S.; Itoh, M.; Osawa, T. Inhibition of Experimental Pulmonary Metastasis of Mouse Colon Adenocarcinoma 26 Sublines by a Sialic Acid: Nucleoside Conjugate Having Sialyltransferase Inhibiting Activity. Cancer Res. 1986, 46, 858–862. [Google Scholar] [PubMed]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Yu, X.; Han, Y.; Wu, Y.; Wang, S.; Chen, X.; Zhang, J.; Wang, S. α2,6-Sialylation promotes immune escape in hepatocarcinoma cells by regulating T cell functions and CD147/MMP signaling. J. Physiol. Biochem. 2019, 75, 199–207. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshioka, Y.; Yamamoto, Y.; Ochiya, T. How cancer cells dictate their microenvironment: Present roles of extracellular vesicles. Cell. Mol. Life Sci. 2017, 74, 697–713. [Google Scholar] [CrossRef]
- Sceneay, J.; Smyth, M.J.; Möller, A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Akagi, T.; Kato, K.; Kobayashi, M.; Kosaka, N.; Ochiya, T.; Ichiki, T. On-Chip Immunoelectrophoresis of Extracellular Vesicles Released from Human Breast Cancer Cells. PLoS ONE 2015, 10, e0123603. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, K.; Zhang, G.; Jia, Z.; Yu, Y.; Guo, J.; Hua, Y.; Guo, F.; Li, X.; Zou, W.; et al. Enrichment of CD44 in Exosomes From Breast Cancer Cells Treated With Doxorubicin Promotes Chemoresistance. Front. Oncol. 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Jiawei, S.; Zhi, C.; Kewei, T.; Xiaoping, L. Magnetic bead-based adsorption strategy for exosome isolation. Front. Bioeng. Biotechnol. 2022, 10, 942077. [Google Scholar] [CrossRef]
- El-Sayes, N.; Vito, A.; Mossman, K. Tumor Heterogeneity: A Great Barrier in the Age of Cancer Immunotherapy. Cancers 2021, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.B.; Knotts, T.A. Communication: Antibody stability and behavior on surfaces. J. Chem. Phys. 2015, 143, 061101. [Google Scholar] [CrossRef] [PubMed]
- Papsidero, L.D.; Wang, M.C.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. A Prostate Antigen in Sera of Prostatic Cancer Patients1. Cancer Res. 1980, 40, 2428–2432. [Google Scholar] [PubMed]
- Wang, M.C.; Papsidero, L.D.; Kuriyama, M.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Prostate antigen: A new potential marker for prostatic cancer. Prostate 1981, 2, 89–96. [Google Scholar] [CrossRef]
- Haga, Y.; Uemura, M.; Baba, S.; Inamura, K.; Takeuchi, K.; Nonomura, N.; Ueda, K. Identification of Multisialylated LacdiNAc Structures as Highly Prostate Cancer Specific Glycan Signatures on PSA. Anal. Chem. 2019, 91, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.; Kuhnle, G.; Spencer, D.I.R.; Osborn, H.M.I. Assays for the identification and quantification of sialic acids: Challenges, opportunities and future perspectives. Biorg. Med. Chem. 2021, 30, 115882. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Uchimura, E.; Koshino, H.; Okano, T.; Kataoka, K. Anomalous Binding Profile of Phenylboronic Acid with N-Acetylneuraminic Acid (Neu5Ac) in Aqueous Solution with Varying pH. J. Am. Chem. Soc. 2003, 125, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Osawa, S.; Arai, T.; Maejima, Y.; Otsuka, H.; Miyahara, Y. Potentiometric Determination of Circulating Glycoproteins by Boronic Acid End-Functionalized Poly(ethylene glycol)-Modified Electrode. Bioconj. Chem. 2021, 32, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Cabral, H.; Sato, N.; Kataoka, K.; Miyahara, Y. Assessment of Tumor Metastasis by the Direct Determination of Cell-Membrane Sialic Acid Expression. Angew. Chem. Int. Ed. 2010, 49, 5494–5497. [Google Scholar] [CrossRef]
- Shashni, B.; Horiguchi, Y.; Kurosu, K.; Furusho, H.; Nagasaki, Y. Application of surface enhanced Raman spectroscopy as a diagnostic system for hypersialylated metastatic cancers. Biomaterials 2017, 134, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Huang, A. Detection of sialic acid using boronic-acid-functionalized metal organic framework UiO-66-NH2@B(OH)2. Talanta 2021, 232, 122434. [Google Scholar] [CrossRef]
- Matsumoto, A.; Stephenson-Brown, A.J.; Khan, T.; Miyazawa, T.; Cabral, H.; Kataoka, K.; Miyahara, Y. Heterocyclic boronic acids display sialic acid selective binding in a hypoxic tumor relevant acidic environmen. Chem. Sci. 2017, 8, 6165–6170. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Barthelmes, K.; Miyahara, Y.; Matsumoto, A. pH-responsive Adsorption and Dissociation of Sialic Acid Expressed Protein on Boronic Acid Immobilized Surface. Chem. Lett. 2021, 50, 1467–1469. [Google Scholar] [CrossRef]
- Roca, A.G.; Costo, R.; Rebolledo, A.F.; Veintemillas-Verdaguer, S.; Tartaj, P.; González-Carreño, T.; Morales, M.P.; Serna, C.J. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224002. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Kurlyandskaya, G.; Litvinova, L.; Safronov, A.; Schupletsova, V.; Tyukova, I.; Khaziakhmatova, O.; Slepchenko, G.; Yurova, K.; Cherempey, E.; Kulesh, N.; et al. Water-Based Suspensions of Iron Oxide Nanoparticles with Electrostatic or Steric Stabilization by Chitosan: Fabrication, Characterization and Biocompatibility. Sensors 2017, 17, 2605. [Google Scholar] [CrossRef]
- Próspero, A.G.; Quini, C.C.; Bakuzis, A.F.; Fidelis-de-Oliveira, P.; Moretto, G.M.; Mello, F.P.F.; Calabresi, M.F.F.; Matos, R.V.R.; Zandoná, E.A.; Zufelato, N.; et al. Real-time in vivo monitoring of magnetic nanoparticles in the bloodstream by AC biosusceptometry. J. Nanobiotechnol. 2017, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y.; et al. Urokinase-Conjugated Magnetite Nanoparticles as a Promising Drug Delivery System for Targeted Thrombolysis: Synthesis and Preclinical Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 36764–36775. [Google Scholar] [CrossRef]
- Nagasaki, Y.; Kobayashi, H.; Katsuyama, Y.; Jomura, T.; Sakura, T. Enhanced immunoresponse of antibody/mixed-PEG co-immobilized surface construction of high-performance immunomagnetic ELISA system. J. Colloid. Interface Sci. 2007, 309, 524–530. [Google Scholar] [CrossRef]
- Kubota, M.; Yoshimoto, K.; Yuan, X.; Nagasaki, Y. Improvement of the thermal stability of streptavidin immobilized on magnetic beads by the construction of a mixed poly(ethylene glycol) tethered-chain layer. Polym. J. 2011, 43, 493–496. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Napoleon, J.V.; Srinivasarao, M.; Singhal, S.; Savran, C.A.; Low, P.S. Efficient capture of circulating tumor cells with low molecular weight folate receptor-specific ligands. Sci. Rep. 2022, 12, 8555. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2023, 20, 2304848. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Huang, Y.; Wang, M.; Cen, C.; Tang, S.; Dique, M.R.; Cai, L.; Luis, M.A.; Smollar, J.; et al. Detection Methods and Clinical Applications of Circulating Tumor Cells in Breast Cancer. Front. Oncol. 2021, 11, 652253. [Google Scholar] [CrossRef]
- Deshayes, S.; Cabral, H.; Ishii, T.; Miura, Y.; Kobayashi, S.; Yamashita, T.; Matsumoto, A.; Miyahara, Y.; Nishiyama, N.; Kataoka, K. Phenylboronic Acid-Installed Polymeric Micelles for Targeting Sialylated Epitopes in Solid Tumors. J. Am. Chem. Soc. 2013, 135, 15501–15507. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiguchi, Y.; Yasuura, M.; Ashiba, H.; Tan, Z.L.; Fukuda, T. Simple Binding and Dissociation of a Sialoglycoprotein Using Boronic Acid-Modified Functional Interfaces on Microparticles. Sensors 2024, 24, 1080. https://doi.org/10.3390/s24041080
Horiguchi Y, Yasuura M, Ashiba H, Tan ZL, Fukuda T. Simple Binding and Dissociation of a Sialoglycoprotein Using Boronic Acid-Modified Functional Interfaces on Microparticles. Sensors. 2024; 24(4):1080. https://doi.org/10.3390/s24041080
Chicago/Turabian StyleHoriguchi, Yukichi, Masato Yasuura, Hiroki Ashiba, Zheng Lin Tan, and Takashi Fukuda. 2024. "Simple Binding and Dissociation of a Sialoglycoprotein Using Boronic Acid-Modified Functional Interfaces on Microparticles" Sensors 24, no. 4: 1080. https://doi.org/10.3390/s24041080