Characterizing Conformational Change of a Thermoresponsive Polymeric Nanoparticle with Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BPME Initiator Assembly onto SNPs
2.3. Surface-Initiated Atom Transfer Radical Polymerization of POEGMA-144
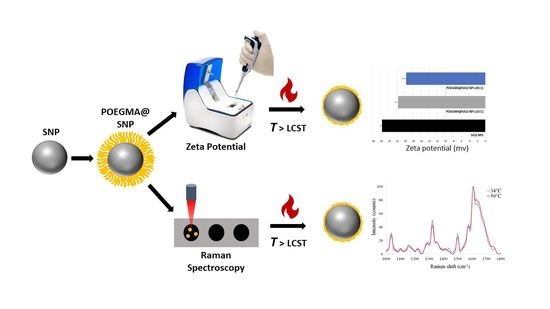
2.4. Instruments for Characterization of POEGMA@SNPs
2.5. Preparation for Raman Spectroscopy and Thermal Heating Apparatus
2.6. Raman Characterization of POEGMA-144 Conformational Change at LCST
2.7. Raman Spectral Processing
2.8. Selection of Raman Peaks Characteristic to POEGMA-144 Conformational Change at LCST
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of POEGMA@SNPs with Zeta Potential Measurements
3.2. Determination of Characteristic Raman Peaks of POEGMA@SNPs
3.3. Raman Detection of Conformational Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. J. Physics Mater. 2019, 2, 032004. [Google Scholar] [CrossRef]
- Ali, N.; Bilal, M.; Khan, A.; Nguyen, T.A.; Gupta, R.K. Chapter Eight—Biomedical applications of smart polymer composites. In Smart Polymer Nanocomposites; Bhawani, S.A., Khan, A., Jawaid, M., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 183–204. [Google Scholar]
- Sano, K.; Umemoto, K.; Miura, H.; Ohno, S.; Iwata, K.; Kawakami, R.; Munekane, M.; Yamasaki, T.; Citterio, D.; Hiruta, Y.; et al. Feasibility of Using Poly[oligo(ethylene glycol) Methyl Ether Methacrylate] as Tumor-Targeted Carriers of Diagnostic Drugs. ACS Appl. Polym. Mater. 2022, 4, 4734–4740. [Google Scholar] [CrossRef]
- Guntnur Vishwanath, R.T. Modulation of Neural Activity via On-Demand Magnetothermal Drug Release. Ph.D. Thesis, The University of Texas at San Antonio, Ann Arbor, MI, USA, 2022; p. 178. [Google Scholar]
- Gao, X.; Kucerka, N.; Nieh, M.-P.; Katsaras, J.; Zhu, S.; Brash, J.L.; Sheardown, H. Chain Conformation of a New Class of PEG-Based Thermoresponsive Polymer Brushes Grafted on Silicon as Determined by Neutron Reflectometry. Langmuir 2009, 25, 10271–10278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guntnur, R.T.; Muzzio, N.; Morales, M.; Romero, G. Phase transition characterization of poly(oligo(ethylene glycol)methyl ether methacrylate) brushes using the quartz crystal microbalance with dissipation. Soft Matter 2021, 17, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Bebis, K.; Jones, M.W.; Haddleton, D.M.; Gibson, M.I. Thermoresponsive behaviour of poly[(oligo(ethyleneglycol methacrylate)]s and their protein conjugates: Importance of concentration and solvent system. Polym. Chem. 2011, 2, 975–982. [Google Scholar] [CrossRef]
- Gibson, M.I.; O’Reilly, R.K. To aggregate, or not to aggregate? Considerations in the design and application of polymeric thermally-responsive nanoparticles. Chem. Soc. Rev. 2013, 42, 7204–7213. [Google Scholar] [CrossRef] [Green Version]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Yoshida, R.; Sakai, K.; Sakurai, Y.; Okano, T. Temperature-responsive shrinking kinetics of poly (N-isopropylacrylamide) copolymer gels with hydrophilic and hydrophobic comonomers. J. Membr. Sci. 1995, 101, 13–22. [Google Scholar] [CrossRef]
- Rosi, B.P.; Tavagnacco, L.; Comez, L.; Sassi, P.; Ricci, M.; Buratti, E.; Bertoldo, M.; Petrillo, C.; Zaccarelli, E.; Chiessi, E.; et al. Thermoresponsivity of poly(N-isopropylacrylamide) microgels in water-trehalose solution and its relation to protein behavior. J. Colloid Interface Sci. 2021, 604, 705–718. [Google Scholar] [CrossRef]
- Luis, T.; Henry, G.; Joshua, C.U.; Maisha, A.; Sean, M.; Rohini, T.G.; Nikolay, A.; Gabriela, R.; Yong, Y.J. Label-free quantification of POEGMA-144 polymerization and thermal response via PC-TIR biosensor. Proc. SPIE 2023, 12391, 99–104. [Google Scholar]
- Liu, P.; Lu, W.; Wang, W.-J.; Li, B.-G.; Ye, Z.; Zhu, S.; Joh; Guire, M.; Nassab, A.; Andrews; et al. Synthesis and Characterization of PE-b-POEGMA Copolymers Prepared by Linear/Hyperbranched Telechelic Polyethylene-Initiated ATRP of Oligo(ethylene glycol) Methacrylates. In Progress in Controlled Radical Polymerization: Materials and Applications; American Chemical Society: Washington, DC, USA, 2012; pp. 39–64. [Google Scholar] [CrossRef]
- Ishizone, T.; Han, S.; Hagiwara, M.; Yokoyama, H. Synthesis and Surface Characterization of Well-Defined Amphiphilic Block Copolymers Containing Poly[oligo(ethylene glycol) methacrylate] Segments. Macromolecules 2006, 39, 962–970. [Google Scholar] [CrossRef]
- Heath, S.; Han, Y.; Hua, R.; Roy, A.; Jiang, J.; Nyman, J.S.; Wang, X. Assessment of glycosaminoglycan content in bone using Raman spectroscopy. Bone 2023, 171, 116751. [Google Scholar] [CrossRef]
- Kotula, A.P.; Snyder, C.R.; Migler, K. Determining conformational order and crystallinity in polycaprolactone via Raman spectroscopy. Polymer 2017, 117, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, X.-D.; Tian, T.; Chu, L.-Q. Incorporation of multilayered silver nanoparticles into polymer brushes as 3-dimensional SERS substrates and their application for bacteria detection. Appl. Surf. Sci. 2017, 407, 185–191. [Google Scholar] [CrossRef]
- Zou, X.-N.; Han, X.; Zhang, Q.; Yin, J.-J.; Chu, L.-Q. Preparation and antibacterial activity of silver-loaded poly(oligo(ethylene glycol) methacrylate) brush. J. Biomater. Sci. Polym. Ed. 2019, 30, 756–768. [Google Scholar] [CrossRef]
- Maeda, Y.; Kitano, H. The structure of water in polymer systems as revealed by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 2433–2446. [Google Scholar] [CrossRef]
- Maeda, Y.; Yamamoto, H.; Ikeda, I. Phase Separation of Aqueous Solutions of Poly(N-isopropylacrylamide) Investigated by Confocal Raman Microscopy. Macromolecules 2003, 36, 5055–5057. [Google Scholar] [CrossRef]
- Pitkänen, L.; Urbas, A.A.; Striegel, A.M. On the feasibility of determining polymer chemical heterogeneity by SEC with continuous off-line Raman detection. Polym. Chem. 2015, 6, 4864–4874. [Google Scholar] [CrossRef]
- Kuzmin, V.; Novikov, V.; Ustynyuk, L.; Prokhorov, K.; Sagitova, E.; Nikolaeva, G. Raman spectra of polyethylene glycols: Comparative experimental and DFT study. J. Mol. Struct. 2020, 1217, 128331. [Google Scholar] [CrossRef]
- Schmälzlin, E.; Moralejo, B.; Rutowska, M.; Monreal-Ibero, A.; Sandin, C.; Tarcea, N.; Popp, J.; Roth, M.M. Raman Imaging with a Fiber-Coupled Multichannel Spectrograph. Sensors 2014, 14, 21968–21980. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Chen, X.; Chen, J.; Zhang, W.; Zhang, M.Q. Observation of mutual diffusion of macromolecules in PS/PMMA binary films by confocal Raman microscopy. Soft Matter 2012, 8, 4780–4787. [Google Scholar] [CrossRef]
- Sharma, N.; Tomar, S.; Shkir, M.; Choubey, R.K.; Singh, A. Study of Optical and Electrical Properties of Graphene Oxide. Mater. Today Proc. 2021, 36, 730–735. [Google Scholar] [CrossRef]
- Feng, W.; Chen, R.; Brash, J.L.; Zhu, S. Surface-Initiated Atom Transfer Radical Polymerization of Oligo(ethylene glycol) Methacrylate: Effect of Solvent on Graft Density. Macromol. Rapid Commun. 2005, 26, 1383–1388. [Google Scholar] [CrossRef]
- Hoffmann, A.; Bienemann, O.; Vieira, I.D.S.; Herres-Pawlis, S. New Guanidine-Pyridine Copper Complexes and Their Application in ATRP. Polymers 2014, 6, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.K.; Min, K.; Matyjaszewski, K. Preparation of Poly(oligo(ethylene glycol) monomethyl ether methacrylate) by Homogeneous Aqueous AGET ATRP. Macromolecules 2006, 39, 3161–3167. [Google Scholar] [CrossRef]
- Wu, L.; Glebe, U.; Böker, A. Surface-initiated controlled radical polymerizations from silica nanoparticles, gold nanocrystals, and bionanoparticles. Polym. Chem. 2015, 6, 5143–5184. [Google Scholar] [CrossRef] [Green Version]
- Piechocki, K.; Kozanecki, M. Hydration in thermo-responsive oligoether methacrylate hydrogels studied by FT-IR spectroscopy. Polymer 2021, 223, 123638. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition. Polymers 2019, 11, 1657. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.-F.; Weichenhan, K.; Akdemir, Ö.; Hoth, A. About the Phase Transitions in Aqueous Solutions of Thermoresponsive Copolymers and Hydrogels Based on 2-(2-methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic, mechanical, and dielectric measurements on complex samples with the quartz crystal microbalance. Phys. Chem. Chem. Phys. 2008, 10, 4516–4534. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trabucco, L.; Heath, S.; Shaw, J.; McFadden, S.; Wang, X.; Ye, J.Y. Characterizing Conformational Change of a Thermoresponsive Polymeric Nanoparticle with Raman Spectroscopy. Sensors 2023, 23, 5713. https://doi.org/10.3390/s23125713
Trabucco L, Heath S, Shaw J, McFadden S, Wang X, Ye JY. Characterizing Conformational Change of a Thermoresponsive Polymeric Nanoparticle with Raman Spectroscopy. Sensors. 2023; 23(12):5713. https://doi.org/10.3390/s23125713
Chicago/Turabian StyleTrabucco, Luis, Savannah Heath, Jonathan Shaw, Sean McFadden, Xiaodu Wang, and Jing Yong Ye. 2023. "Characterizing Conformational Change of a Thermoresponsive Polymeric Nanoparticle with Raman Spectroscopy" Sensors 23, no. 12: 5713. https://doi.org/10.3390/s23125713