In Situ Oxidation of Cu2O Crystal for Electrochemical Detection of Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
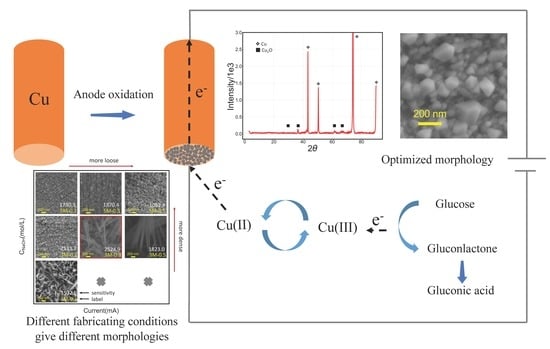
2.2. Preparation of the Cu2O Electrode
2.3. Characterization Apparatus
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of the Cu2O Glucose Electrode
3.2. Electrochemical Behavior of the Cu2O Electrode
3.3. Amperometric Detection of Glucose
3.4. Stability and Selectivity
3.5. Mechanism of In Situ Deposition of Cu2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weiss, R.; Yegorchikov, Y.; Shusterman, A.; Raz, I. Noninvasive Continuous Glucose Monitoring Using Photoacoustic Technology—Results from the First 62 Subjects. Diabetes Technol. Ther. 2007, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Guerrasantos, L.; Käppeli, O.; Fiechter, A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl. Environ. Microbiol. 1984, 48, 301–305. [Google Scholar] [Green Version]
- Murphy, A.P.; Henthorne, L.R. One-Step Synthesis of Vitamin-C (l-Ascorbic Acid). U.S. Patent 5998634A, 15 March 1999. [Google Scholar]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.-S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Liu, N.; Ma, Z. A new luminol chemiluminescence sensor for glucose based on pH-dependent graphene oxide. Analyst 2013, 138, 4393–4397. [Google Scholar] [CrossRef]
- Zhou, S.; Min, X.; Dou, H.; Sun, K.; Chen, C.-Y.; Chen, C.-T.; Zhang, Z.; Jin, Y.; Shen, Z. Facile fabrication of dextran-based fluorescent nanogels as potential glucose sensors. Chem. Commun. 2013, 49, 9473–9475. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.F.; Ruchti, T.L.; Blank, T.B.; Thennadil, S.N.; Monfre, S.L. Noninvasive prediction of glucose by near-infrared diffuse reflectance spectroscopy. Clin. Chem. 1999, 45, 1651–1658. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 2006, 102, 29–45. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Si, P.; Ding, S.; Yuan, J.; Lou, X.W.; Kim, D.H. Hierarchically structured one-dimensional TiO2 for protein immobilization, direct electrochemistry, and mediator-free glucose sensing. ACS Nano 2011, 5, 7617–7626. [Google Scholar] [CrossRef]
- Alwarappan, S.; Singh, S.R.; Pillai, S.; Kumar, A.; Mohapatra, S. Direct electrochemistry of glucose oxidase at a gold electrode modified with graphene nanosheets. Anal. Lett. 2012, 45, 746–753. [Google Scholar] [CrossRef]
- Alwarappan, S.; Liu, C.; Kumar, A.; Li, C.-Z. Enzyme-doped graphene nanosheets for enhanced glucose biosensing. J. Phys. Chem. C 2010, 114, 12920–12924. [Google Scholar] [CrossRef]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Z.; Chen, D.-J.; Jia, T.; Cai, Z.; Wang, X.; Chen, X.; Chen, G.; Oyama, M. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens. Bioelectron. 2010, 25, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Gao, R. Ti/TiO2 nanotube array/Ni composite electrodes for nonenzymatic amperometric glucose sensing. J. Phys. Chem. C 2010, 114, 4408–4413. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Zheng, W.; Yang, J.; Zhang, H.; Wang, C. Electrospun palladium (IV)-doped copper oxide composite nanofibers for non-enzymatic glucose sensors. Electrochem. Commun. 2009, 11, 1811–1814. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, C.; Xu, C.; Liu, P.; Xu, X.; Ding, Y. Aerobic oxidation of D-glucose on support-free nanoporous gold. J. Phys. Chem. C 2008, 112, 9673–9678. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 2007, 363, 143–150. [Google Scholar] [CrossRef]
- Luo, P.F.; Kuwana, T. Nickel-titanium alloy electrode as a sensitive and stable LCEC detector for carbohydrates. Anal. Chem. 1994, 66, 2775–2782. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, F.; Yu, J.; Hu, S. Electro-oxidation of glucose at self-assembled monolayers incorporated by copper particles. Talanta 2006, 70, 449–454. [Google Scholar] [CrossRef]
- Zaidi, S.A.; Shin, J.H. Recent developments in nanostructure based electrochemical glucose sensors. Talanta 2016, 149, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guo, H.; Wang, Z.; Long, Y.; Li, W.; Tu, Y. Au@Cu2O core-shell structure for high sensitive non-enzymatic glucose sensor. Sens. Actuators B Chem. 2018, 255, 2510–2519. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, L.; Manuzzi, D.; de los Monteros, H.V.E.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens. Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Reitz, E.; Jia, W.; Gentile, M.; Wang, Y.; Lei, Y. CuO nanospheres based nonenzymatic glucose sensor. Electroanalysis 2008, 20, 2482–2486. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Kaur, B.; Srivastava, R. Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J. Colloid Interface Sci. 2012, 370, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, L.; Zhang, Y.; Zhao, H.; Li, X.; Li, Y.; Wen, L.; Yan, Z.; Huo, Z. Hyper-Branched Cu@Cu2O Coaxial Nanowires Mesh Electrode for Ultra-Sensitive Glucose Detection. ACS Appl. Mater. Interfaces 2015, 7, 16802–16812. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, C.; Liu, H.; Du, G.; He, X.; Xi, Y. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2010, 144, 220–225. [Google Scholar] [CrossRef]
- Babu, T.G.S.; Ramachandran, T. Development of highly sensitive non-enzymatic sensor for the selective determination of glucose and fabrication of a working model. Electrochim. Acta 2010, 55, 1612–1618. [Google Scholar] [CrossRef]
- Luo, Z.J.; Han, T.T.; Qu, L.L.; Wu, X.Y. A ultrasensitive nonenzymatic glucose sensor based on Cu2O polyhedrons modified Cu electrode. Chin. Chem. Lett. 2012, 23, 953–956. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.; Song, Y. A facile strategy to prepare Cu2O/Cu electrode as a sensitive enzyme-free glucose sensor. Int. J. Electrochem. Sci. 2012, 7, 12587–12600. [Google Scholar]
- Babu, T.G.S.; Ramachandran, T.; Nair, B. Single step modification of copper electrode for the highly sensitive and selective non-enzymatic determination of glucose. Microchim. Acta 2010, 169, 49–55. [Google Scholar] [CrossRef]
- Hovancová, J.; Šišoláková, I.; Oriňaková, R.; Oriňak, A. Nanomaterial-based electrochemical sensors for detection of glucose and insulin. J. Solid State Electrochem. 2017, 21, 2147–2166. [Google Scholar] [CrossRef]
- Gnana kumar, G.; Amala, G.; Gowtham, S.M. Recent advancements, key challenges and solutions in non-enzymatic electrochemical glucose sensors based on graphene platforms. RSC Adv. 2017, 7, 36949–36976. [Google Scholar] [CrossRef] [Green Version]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187–1197. [Google Scholar] [CrossRef]
- Wei, H.; Sun, J.-J.; Guo, L.; Li, X.; Chen, G.-N. Highly enhanced electrocatalytic oxidation of glucose and shikimic acid at a disposable electrically heated oxide covered copper electrode. Chem. Commun. 2009, 20, 2842–2844. [Google Scholar] [CrossRef] [PubMed]
- Sargent, F. A study of the normal distribution of ascorbic acid between the red cells and plasma of human blood. J. Biol. Chem 1947, 171, 471–476. [Google Scholar]
- Ambade, V.; Arora, M.M.; Singh, P.; Somani, B.L.; Basannar, D. Adrenaline, noradrenaline and dopamine level estimation in depression: Does it help? Med. J. Armed Forces India 2009, 65, 216–220. [Google Scholar] [CrossRef]
- So, A.; Thorens, B. Science in medicine Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef]
- Arthur, P.G.; Kent, J.C.; Potter, J.M.; Hartmann, P.E. Lactose in blood in nonpregnant, pregnant, and lactating women. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 254–259. [Google Scholar] [CrossRef]
- Tang, L.; Lv, J.; Kong, C.; Yang, Z.; Li, J. Facet-dependent nonenzymatic glucose sensing properties of Cu2O cubes and octahedra. New J. Chem. 2016, 40, 6573–6576. [Google Scholar] [CrossRef]
- Yazid, S.N.A.M.; Isa, I.M.; Hashim, N. Novel alkaline-reduced cuprous oxide/graphene nanocomposites for non-enzymatic amperometric glucose sensor application. Mater. Sci. Eng. C 2016, 68, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, F.; Chen, Z.; Qiu, Y.; Zhai, T.; Hu, M.; Zhang, C.; Huang, K. Flower-like MoS2decorated with Cu2O nanoparticles for non-enzymatic amperometric sensing of glucose. Talanta 2017, 167, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, Y.; Zhang, Y.; Weng, W.; Li, S. Carbon quantum dots/octahedral Cu2O nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sens. Actuators B Chem. 2015, 206, 735–743. [Google Scholar] [CrossRef]
- Long, M.; Tan, L.; Liu, H.; He, Z.; Tang, A. Novel helical TiO2 nanotube arrays modified by Cu2O for enzyme-free glucose oxidation. Biosens. Bioelectron. 2014, 59, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, A.; Li, H.; Wang, L.; Li, S.; Kong, J.; Bao, X.; Yang, R. A non-enzymatic glucose sensing based on hollow cuprous oxide nanospheres in a Nafion matrix. Sens. Actuators B Chem. 2015, 214, 169–173. [Google Scholar] [CrossRef]
- Yin, H.; Cui, Z.; Wang, L.; Nie, Q. In situ reduction of the Cu/Cu2O/carbon spheres composite for enzymaticless glucose sensors. Sens. Actuators B Chem. 2016, 222, 1018–1023. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, X.; Li, Z.; Zheng, X.; Zheng, J. Synthesis of Cu2O on AlOOH/reduced graphene oxide for non-enzymatic amperometric glucose sensing. Anal. Methods 2016, 8, 1527–1531. [Google Scholar] [CrossRef]
- Zhou, D.L.; Feng, J.J.; Cai, L.Y.; Fang, Q.X.; Chen, J.R.; Wang, A.J. Facile synthesis of monodisperse porous Cu2O nanospheres on reduced graphene oxide for non-enzymatic amperometric glucose sensing. Electrochim. Acta 2014, 115, 103–108. [Google Scholar] [CrossRef]
- Neetzel, C.; Muench, F.; Matsutani, T.; Jaud, J.C.; Broetz, J.; Ohgai, T.; Ensinger, W. Facile wet-chemical synthesis of differently shaped cuprous oxide particles and a thin film: Effect of catalyst morphology on the glucose sensing performance. Sens. Actuators B Chem. 2015, 214, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Khedekar, V.V.; Bhanage, B.M. Simple Electrochemical Synthesis of Cuprous Oxide Nanoparticles and Their Application as a Non-Enzymatic Glucose Sensor. J. Electrochem. Soc. 2016, 163, B248–B251. [Google Scholar] [CrossRef]
- He, J.; Jiang, Y.; Peng, J.; Li, C.; Yan, B.; Wang, X. Fast synthesis of hierarchical cuprous oxide for nonenzymatic glucose biosensors with enhanced sensitivity. J. Mater. Sci. 2016, 51, 9696–9704. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.; Song, B.; Liu, J.; Hu, C.; Wei, D.; Wong, C.P. Precisely quantified catalyst based on in situ growth of Cu2O nanoparticles on a graphene 3D network for highly sensitive glucose sensor. Sens. Actuators B Chem. 2017, 250, 333–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, F.; Sun, Y.; Shi, Y.; Wen, Z.; Li, Z. Assembly of Ni(OH)2 nanoplates on reduced graphene oxide: A two dimensional nanocomposite for enzyme-free glucose sensing. J. Mater. Chem. 2011, 21, 16949–16954. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, P.; Li, H.; Chen, S. Controllable growth of cobalt oxide nanoparticles on reduced graphene oxide and its application for highly sensitive glucose sensor. Int. J. Electrochem. Sci. 2014, 9, 7369–7381. [Google Scholar]
- Heidari, H.; Habibi, E. Amperometric enzyme-free glucose sensor based on the use of a reduced graphene oxide paste electrode modified with electrodeposited cobalt oxide nanoparticles. Microchim. Acta 2016, 183, 2259–2266. [Google Scholar] [CrossRef]
- Belkhalfa, H.; Teodorescu, F.; Quéniat, G.; Coffinier, Y.; Dokhan, N.; Sam, S.; Abderrahmani, A.; Boukherroub, R.; Szunerits, S. Insulin impregnated reduced graphene oxide/Ni(OH)2 thin films for electrochemical insulin release and glucose sensing. Sens. Actuators B Chem. 2016, 237, 693–701. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S. Nanoparticles-assembled NiO nanosheets templated by graphene oxide film for highly sensitive non-enzymatic glucose sensing. Sens. Actuators B Chem. 2017, 238, 788–794. [Google Scholar] [CrossRef]
- Lv, W.; Jin, F.M.; Guo, Q.; Yang, Q.H.; Kang, F. DNA-dispersed graphene/NiO hybrid materials for highly sensitive non-enzymatic glucose sensor. Electrochim. Acta 2012, 73, 129–135. [Google Scholar] [CrossRef]
- Guilbeau, E.J.; Towe, B.C.; Muehlbauer, M.J. Model for a Thermoelectric Enzyme Glucose Sensor. Anal. Chem. 1989, 61, 77–83. [Google Scholar]
- Schmidtke, D.W.; Freeland, A.C.; Heller, A.; Bonnecaze, R.T. Measurement and modeling of the transient difference between blood and subcutaneous glucose concentrations in the rat after injection of insulin. Proc. Natl. Acad. Sci. USA 1998, 95, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablecki, M.; Gough, D.A. Simulations of the frequency response of implantable glucose sensors. Anal. Chem. 2000, 72, 1853–1859. [Google Scholar] [CrossRef] [PubMed]




| Electrode | Sensitivity (μA·mM−1·cm−2) | Linear Range (mM) | Detection Limit (μM) | Response Time (s) | Cycles (times) | Ref. |
|---|---|---|---|---|---|---|
| Octahedral Cu2O | 293.893 | 0.1–5 | 5.11 | 5 | 7 | [41] |
| Cu2O/graphene | 1330.05 | 0.01–3.0 | 0.36 | 7 | 7 | [42] |
| Cu2O/MoS2 | 3108.87 | 0.01–4.0 | 1 | 3 | 10 | [43] |
| CQDs/O-Cu2O | 298 | 0.02–4.3 | 8.4 | 10 | 3 | [44] |
| Cu2O/TiO2 nanotube | 14.56 | 3.0–9.0 | 62 | 3 | 5 | [45] |
| rGOs wrapped Cu2O | 285 | 0.3–3.3 | 3.3 | 9 | 1 | [46] |
| Hollow Cu2O | 2038.2 | 0.00125–0.0375 | 0.41 | 3 | 5 | [47] |
| Cu/Cu2O/CS | 63.8 | 0.01–0.69 | 5 | 5 | 1 | [48] |
| Cu2O/AlOOH/rGO | 155.1 | 0.005–14.77 | 2.6 | 5 | 3 | [49] |
| rGOs-porous Cu2O | 185.1 | 0.01–6 | 0.05 | 3 | 1 | [50] |
| Au@Cu2O | 715 | 0.05–2.0 | 18 | 20 | 5 | [23] |
| polyhedral Cu2O | 300.96 | 1.2–298 | 0.144 | 4 | 1 | [51] |
| Cu2O/PC platinum | 507 | 0.1–2.5 | 26 | 5 | 3 | [52] |
| DH Cu2O/GCE | 1231.7 | 0.019–1.089 | 18.5 | 3 | 2 | [53] |
| Nafion/Cu@Cu2O/GCE | 1420 | 0.0007–2.0 | 40 nm | 2 | 7 | [27] |
| Cu2O NPs/G3DN/CP | 2310 | 0.00048–1.813 | 0.14 | 1.6 | 4 | [54] |
| Cu2O/Cu | 62.29 | 0.05–6.75 | 37 | 5 | 2 | [31] |
| Cu2O/Cu | 728.67 | 0.01–7.53 | 3 | 3.6 | 1 | [30] |
| CuO/Cu | 761.9 | 0.002–20 | 2 | 1 | 7 | [32] |
| CuO/CuOx/Cu | 1890 | 0.002–15 | 0.05 | 1 | 6 | [29] |
| Co3O4/GOH/GCE | 492.8 | 0.25–10 | - | 8 | 1 | [55] |
| Co3O4/rGO/GCE | 1366 | 0.0005–1.277 | 0.18 | 2 | 2 | [56] |
| Co3O4/rGOP | 1.21 | 0.04–4 | 1.4 | - | 1 | [57] |
| Ni(OH)2/insulin/rGO/Au | 18.9 | 0.005–10 | 5 | 7 | 2 | [58] |
| NiO/rGO/GCE | 1138 | 0.001–0.4 | 0.18 | 2 | 10 | [59] |
| NiO/DNA/graphene/GCE | 9 | 0.001–8 | 2.5 | 8 | 10 | [60] |
| Cu2O@Cu | 2524.9 | 0.1–1.0 | 2.57 | 3 | 10 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Li, Z.; Ren, L.; Su, N.; Lu, D.; Liu, Z. In Situ Oxidation of Cu2O Crystal for Electrochemical Detection of Glucose. Sensors 2019, 19, 2926. https://doi.org/10.3390/s19132926
Lu C, Li Z, Ren L, Su N, Lu D, Liu Z. In Situ Oxidation of Cu2O Crystal for Electrochemical Detection of Glucose. Sensors. 2019; 19(13):2926. https://doi.org/10.3390/s19132926
Chicago/Turabian StyleLu, Chenlin, Zhipeng Li, Liwei Ren, Nan Su, Diannan Lu, and Zheng Liu. 2019. "In Situ Oxidation of Cu2O Crystal for Electrochemical Detection of Glucose" Sensors 19, no. 13: 2926. https://doi.org/10.3390/s19132926