Diversity of Rotifera (Subclass: Monogononta) from Inland Water Bodies in Greece: An Updated Checklist
Abstract
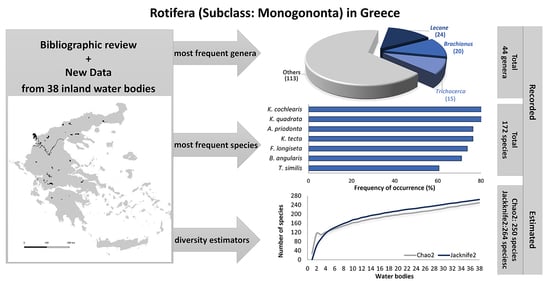
:1. Introduction
2. Materials and Methods
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of biodiversity and ecosystem services by ecological restoration: A meta-analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.M.; Aronson, J.; Newton, A.C.; Pywell, R.F.; Rey-Benayas, J.M. Restoration of ecosystem services and biodiversity: Conflicts and opportunities. Trends Ecol. Evol. 2011, 26, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Gómez, A.; Hagiwara, A.; Galindo, K.H.; Jersabek, C.D.; Malekzadeh-Viayeh, R.; Leasi, F.; Lee, J.S.; et al. Fifteen species in one: Deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 2017, 796, 39–58. [Google Scholar] [CrossRef] [Green Version]
- Papakostas, S.; Michaloudi, E.; Proios, K.; Brehm, M.; Verhage, L.; Rota, J.; Peña, C.; Stamou, G.; Pritchard, V.L.; Fontaneto, D.; et al. Integrative taxonomy recognizes evolutionary units despite widespread mitonuclear discordance: Evidence from a rotifer cryptic species complex. Syst. Biol. 2016, 65, 508–524. [Google Scholar] [CrossRef] [Green Version]
- Michaloudi, E.; Papakostas, S.; Stamou, G.; Neděla, V.; Tihlaříková, E.; Zhang, W.; Declerck, S.A. Reverse taxonomy applied to the Brachionus calyciflorus cryptic species complex: Morphometric analysis confirms species delimitations revealed by molecular phylogenetic analysis and allows the (re) description of four species. PLoS ONE 2018, 13, e0203168. [Google Scholar] [CrossRef]
- Obertegger, U.; Fontaneto, D.; Flaim, G. Using DNA taxonomy to investigate the ecological determinants of plankton diversity: Explaining the occurrence of Synchaeta spp. (Rotifera, Monogononta) in mountain lakes. Freshw. Biol. 2012, 57, 1545–1553. [Google Scholar] [CrossRef]
- Obertegger, U.; Flaim, G.; Fontaneto, D. Cryptic diversity within the rotifer Polyarthra dolichoptera along an altitudinal gradient. Freshw. Biol. 2014, 59, 2413–2427. [Google Scholar] [CrossRef]
- Cieplinski, A.; Weisse, T.; Obertegger, U. High diversity in Keratella cochlearis (Rotifera, Monogononta): Morphological and genetic evidence. Hydrobiologia 2017, 796, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Geburzi, J.C.; McCarthy, M.L. How do they do it?–Understanding the success of marine invasive species. In YOUMARES 8–Oceans Across Boundaries: Learning from Each Other; Jungblut, S., Liebich, V., Bode, M., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 109–124. [Google Scholar]
- Walsh, J.R.; Carpenter, S.R.; Zanden, M.J.V. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc. Natl. Acad. Sci. USA 2016, 113, 4081–4085. [Google Scholar] [CrossRef] [Green Version]
- David, P.; Thebault, E.; Anneville, O.; Duyck, P.F.; Chapuis, E.; Loeuille, N. Impacts of invasive species on food webs: A review of empirical data. Adv. Ecol Res. 2017, 56, 1–60. [Google Scholar]
- Jeppesen, E.; Beklioğlu, M.; Özkan, K.; Akyürek, Z. Salinization increase due to climate change will have substantial negative effects on inland waters: A call for multifaceted research at the local and global scale. Innovation 2020, 1, 100030. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.L. Rotifers: Exquisite metazoans. Integr. Comp. Biol. 2002, 42, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, H. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 2008, 595, 49–59. [Google Scholar] [CrossRef]
- Segers, H. The nomenclature of the Rotifera: Annotated checklist of valid familyand genus-group names. J. Nat. Hist. 2002, 36, 631–640. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Leitner, M.F. The Rotifer World Catalog. World Wide Web Electronic Publication. 2013. Available online: http://www.rotifera.hausdernatur.at/ (accessed on 30 January 2022).
- Allan, J.D. Life history patterns in zooplankton. Am. Nat. 1976, 110, 165–180. [Google Scholar] [CrossRef]
- Wallace, R.L.; Snell, T.W.; Smith, H.A. Chapter 13-Phylum Rotifera. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 225–271. [Google Scholar]
- García-Morales, A.E.; Elías-Gutiérrez, M. DNA barcoding of freshwater Rotifera in Mexico: Evidence of cryptic speciation in common rotifers. Mol. Ecol. Resour. 2013, 13, 1097–1107. [Google Scholar] [CrossRef]
- Havel, J.E.; Shurin, J.B. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnol. Oceanogr. 2004, 49, 1229–1238. [Google Scholar] [CrossRef]
- Segers, H.; De Smet, W.H.; Fischer, C.; Fontaneto, D.; Michaloudi, E.; Wallace, R.L.; Jersabek, C.D. Towards a list of available names in zoology, partim Phylum Rotifera. Zootaxa 2012, 3179, 61–68. [Google Scholar] [CrossRef]
- Jersabek, C.D.; De Smet, W.H.; Hinz, C.; Fontaneto, D.; Hussey, C.G.; Michaloudi, E.; Wallace, R.L.; Segers, H. List of Available Names in Zoology, Candidate Part Phylum Rotifera, genus-group names established before 1 January 2000. 2018a. Available online: https://archive.org/details/LANCandidatePartGeneraRotifera (accessed on 30 January 2022).
- Jersabek, C.D.; De Smet, W.H.; Hinz, C.; Fontaneto, D.; Hussey, C.G.; Michaloudi, E.; Wallace, R.L.; Segers, H. List of Available Names in Zoology, Candidate Part Phylum Rotifera, species-group names established before 1 January 2000. 2018b. Available online: https://archive.org/details/LANCandidatePartSpeciesRotifera (accessed on 30 January 2022).
- ICZN (International Commission on Zoological Nomenclature). Opinion 2430–Parts of the List of Available Names in Zoology for phylum Rotifera: Accepted. Bull. Zool. Nomencl. 2019, 76, 74–76. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Challenges for taxonomy. Nature 2002, 417, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Richard, J. Animaux inférieurs, notamment Entomostracés, recueillis par M. le Prof. Steindachner dans les lacs de la Macédoine. Ann. K. R. Nat. Hist. Hofmus. Wien. 1892, 7, 151–153. [Google Scholar]
- Richard, J. Entomostracés, recueillis par M. le Directeur Steindachner dans les lacs de Janina et de Scutari. Ann. Naturh. Mus. Wien. 1897, 12, 63–66. [Google Scholar]
- Berzinš, B. Liste des Rotiféres provenant du lac Kourna, île de Crète (Grèce). Fragm. Balc. 1956, 1, 207–208. [Google Scholar]
- De Ridder, M. Radierdieren vit Griekland. Biol. Jaarb. 1967, 85, 188–196. [Google Scholar]
- Donner, P.J. Rotatorien aus einigen Auböden der Donau, aus ostmediterranen Böden und aus Kiew. Veröffentlichungen der Arbeitsgemeinschaft Donauforschung 1971, 4, 352–376. [Google Scholar] [CrossRef]
- Zarfdjian, M.H.; Economidis, P.S. Listes provisoires des Rotifères, Cladocéres et Copépodes des eaux continentals Grecques. Biologia Gallo-Hellenica 1989, 15, 129–146. [Google Scholar]
- Ciros-Pérez, J.; Carmona, M.J.; Serra, M. Resource competition between sympatric sibling rotifer species. Limnol. Oceanogr. 2001, 46, 1511–1523. [Google Scholar] [CrossRef] [Green Version]
- Michaloudi, E.; Mills, S.; Papakostas, S.; Stelzer, C.P.; Triantafyllidis, A.; Kappas, I.; Vasileiadou, K.; Proios, K.; Abatzopoulos, T.J. Morphological and taxonomic demarcation of Brachionus asplanchnoidis Charin within the Brachionus plicatilis cryptic species complex (Rotifera, Monogononta). Hydrobiologia 2017, 796, 19–37. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS_9.1_Windows. Available online: https://osf.io/su57f/ (accessed on 14 February 2022).
- Segers, H.; De Smet, W.H. Diversity and endemism in Rotifera: A review, and Keratella Bory de St Vincent. Biodivers Conserv. 2008, 17, 303–316. [Google Scholar] [CrossRef]
- Fontaneto, D.; Barbosa, A.M.; Segers, H.; Pautasso, M. The ‘rotiferologist’effect and other global correlates of species richness in monogonont rotifers. Ecography 2012, 35, 174–182. [Google Scholar] [CrossRef]
- Shumka, S. Checklist of rotifer species from Albania (phylum Rotifera). Opusc. Zool. Inst. Zoosyst. Oecol. Univ. Bp. 2021, 52, 99–109. [Google Scholar] [CrossRef]
- López, C.; Soto, L.M.; Lafuente, W.; Stamou, G.; Michaloudi, E.; Papakostas, S.; Fontaneto, D. Rotifers from inland water bodies of continental Ecuador and Galápagos Islands: An updated checklist. Zootaxa 2020, 4768, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.S.S.; Jiménez-Santos, M.A.; Nandini, S. Rotifer Species Diversity in Mexico: An Updated Checklist. Diversity 2021, 13, 291. [Google Scholar] [CrossRef]
- Fontaneto, D.; Bertani, I.; Cancellario, T.; Rossetti, G.; Obertegger, U. The new Checklist of the Italian Fauna: Rotifera. Biogeogr. –J. Integr. Biogeogr. 2022, 37. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- García-Chicote, J.; Armengol, X.; Rojo, C. Zooplankton species as indicators of trophic state in reservoirs from Mediterranean river basins. Inland Waters 2019, 9, 113–123. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia 2019, 844, 83–103. [Google Scholar] [CrossRef]
- Fontaneto, D.; De Smet, W.H.; Ricci, C. Rotifers in saltwater environments, re-evaluation of an inconspicuous taxon. J. Mar. Biol. Assoc. UK 2006, 86, 623–656. [Google Scholar] [CrossRef]
- Fontaneto, D. Molecular phylogenies as a tool to understand diversity in rotifers. Int. Rev. Hydrobiol. 2014, 99, 178–187. [Google Scholar] [CrossRef]
- Schröder, T.; Walsh, E.J. Cryptic speciation in the cosmopolitan Epiphanes senta complex (Monogononta, Rotifera) with the description of new species. Hydrobiologia 2007, 593, 129–140. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Moustaka-Gouni, M.; Michaloudi, E.; Bobori, D.C. Biogeographical patterns of freshwater micro-and macroorganisms: A comparison between phytoplankton, zooplankton and fish in the eastern Mediterranean. J. Biogeogr. 2010, 37, 1341–1351. [Google Scholar] [CrossRef]
- Proios, K.; Michaloudi, E.; Papakostas, S.; Kappas, I.; Vasileiadou, K.; Abatzopoulos, T.J. Updating the description and taxonomic status of Brachionus sessilis Varga, 1951 (Rotifera: Brachionidae) based on detailed morphological analysis and molecular data. Zootaxa 2014, 3873, 345–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W. Unraveling Hidden Biodiversity: An Example of Integrative Taxonomy Applied to the Hybridizing Brachionus calyciflorus Species Complex. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2021. [Google Scholar]
- Michaloudi, E.; Zarfdjian, M.; Economidis, P.S. The zooplankton of lake Mikri Prespa. Hydrobiologia 1997, 361, 77–94. [Google Scholar] [CrossRef]



| Species | Where |
|---|---|
| Anuraeopsis fissa (Gosse, 1851) | CCh, Doi, Ism, Kas, Kne, Kor, LAU, MgP, MkP, Pam, Pet, Thi, Tri, Veg, Vis, Vol, Vou, Yli |
| Ascomorpha ecaudis Perty, 1850 | Ali, CCh, Kas, Lad, MkP, Pam, Pet, Vol, Vou, Yli, Zaz |
| Ascomorpha ovalis (Bergendal, 1892) | Kre, Par, Thi, Tri |
| Ascomorpha saltans Bartsch, 1870 | Doi, Kas, Kou, Kre, MkP, Par, Veg, Vol, Vou |
| Asplanchna brightwellii Gosse, 1850 | Doi, Kor, Kou |
| Asplanchna girodi Guerne, 1888 | Chi, Doi, Kas, Pet Veg, Vol, Vou, Zaz |
| Asplanchna priodonta Gosse, 1850 | Ali, Amv, CCh, Chi, Ism, Kas, Ker, Kre, Kor, Kou, Lad, Lys, MgP, MkP, Oze, Pam, Par, Pet, Pin, Smo, Str, Sty, Tav, Thi, Tri, Veg, Vol, Yli, Zaz |
| Asplanchna sieboldii (Leydig, 1854) | Ism, Kar, Kas, Lys, Veg, Vou |
| Brachionus angularis Gosse, 1851 | Ali, Amv, CCh, Chi, Doi, Ism, Kar, Kas, KNe, Kor, LAU, Lys, MgP, MkP, Oze, Pam, Par, Pet, Smo, Thi, Tri, Veg, Vis, Vol, Vou, Yli, Zaz |
| Brachionus asplanchnoidis Charin, 1947 | Kor |
| Brachionus bidentatus Anderson, 1889 | Kas |
| Brachionus budapestinensis Daday, 1885 | Ism, LAU, Vol, Vou |
| Brachionus calyciflorus Pallas, 1766 | CCh, Kor, Vol, KNe |
| Brachionus calyciflorus group * | Amv, MgP, Pam, Str, Tav, Tri, Veg, Vis, Yli, Zaz |
| Brachionus cf. caudatus Barrois & Daday, 1894 | Lad |
| Brachionus dorcas Gosse, 1851 | Lys, Oze |
| Brachionus dimidiatus Bryce, 1931 | Doi, Kas, Kor, Vol |
| Brachionus diversicornis Daday, 1883 | Amv, CCh, Chi, Doi, Ism, Kas, Ker, Kor, Kre, LAU, Lys, MgP, MkP, Oze, Pam, Pet, Tav, Tri, Veg, Vol, Vou, Zaz |
| Brachionus elevatus Michaloudi, Papakostas, Stamou et al., 2018 | Chi, Doi, Kar, Kas, Ker, Kor, Lys, Pet, Vou |
| Brachionus falcatus Zacharias, 1898 | Ism, Lys, Tri, Vou |
| Brachionus fernandoi Michaloudi, Papakostas, Stamou et al., 2018 | Ism, LAU, Pet |
| Brachionus forficula Wierzejski, 1891 | Chi, Ism, Kar, Kas, LAU, MkP, Pam, Pet, Thi, Vou, Zaz |
| Brachionus ibericus Ciros-Pérez, Gómez, Serra, 2001 | Ism, Kor, Pik, Vou |
| Brachionus plicatilis Müller, 1786 | Kar, Kor, Pik |
| Brachionus plicatilis group * | Kar, Kor, Mpo, Pik, Smo, Thi |
| Brachionus quadridentatus Hermann, 1783 | Ali, CCh, Ism, Kas, Kor, LAU, MgP, Pik, Sty, Vol, Vou, Zaz |
| Brachionus rubens Ehrenberg, 1838 | Chi, Kor |
| Brachionus sessilis Varga, 1951 | Doi, Par |
| Brachionus urceolaris Müller, 1773 | Ali, CCh, Chi, Ism, Kar, Kas, Kor, LAU, MkP, Zaz |
| Brachionus variabilis Hempel, 1896 | Kar, Kor, Oze, Par, Veg |
| Cephalodella catellina (Muller, 1786) | Ali, Kor, Pik |
| Cephalodella exigua (Gosse, 1886) | Kou |
| Cephalodella forficula (Ehrenberg, 1838) | Vol |
| Cephalodella gibba (Ehrenberg, 1830) | Ali, CCh, Kar, Kas, Kor, Kou, LAU, Lad, MgP, MkP, Vou |
| Cephalodella hiulca Myers, 1924 | Kor |
| Cephalodella licina Wulfert, 1961 | KNe, LAU |
| Cephalodella misgurnus Wulfert, 1937 | Pik |
| Cephalodella stenroosi Wulfert, 1937 | KNe |
| Cephalodella xenica Myers, 1924 | Vou |
| Collotheca libera (Zacharias, 1894) | Vol |
| Collotheca mutabilis (Hudson, 1885) | Vol |
| Colurella adriatica Ehrenberg, 1831 | Ali, Kor, Kou, Vol, Zaz |
| Colurella anodonta Carlin, 1939 | LAU |
| Colurella colurus (Ehrenberg, 1830) | Ali |
| Colurella obtusa (Gosse, 1886) | Kou, Sty, Vol |
| Colurella salina Althaus, 1957 | Vou |
| Colurella uncinata (Müller, 1773) | Ali, Kas, KNe, Kou, LAU, Zaz |
| Conochilus dossuarius Hudson, 1885 | Kas, Veg, Vol, Vou |
| Conochilus hippocrepis (Schrank, 1803) | CCh, MkP |
| Conochilus unicornis Rousselet, 1892 | Amv, Chi, Lys, Oze, Str, Tri, Zaz |
| Dicranophorus hercules Wiszniewski, 1932 | Lad |
| Dicranophorus forcipatus (Müller, 1786) | Sty |
| Dicranophorus grandis (Ehrenberg, 1832) | Kas, LAU |
| Dicranophorus luetkeni (Bergendal, 1892) | Ali |
| Encentrum putorius Wulfert, 1936 | Ali |
| Encentrum uncinatum (Milne, 1886) | Ali |
| Eosphora anthadis Harring & Myers, 1922 | CCh |
| Eosphora ehrenbergi Weber, 1918 | Kas, Kor, Pik |
| Eothinia elongata (Ehrenberg, 1832) | Kou, Vou |
| Epiphanes brachionus (Ehrenberg, 1837) | MgP |
| Epiphanes macroura (Barrois & Daday, 1894) | Chi, Kor, Lys, Vou, Zaz |
| Epiphanes senta (Müller, 1773) | Tav |
| Euchlanis deflexa Gosse, 1851 | Ali |
| Euchlanis dilatata Ehrenberg, 1830 | Ali, Chi, Ism, Kas, Kor, Lad, MkP, Pam, Par, Pet, Tav, Tri, Veg, Vol, Vou, Zaz |
| Euchlanis lyra Hudson, 1886 | Ali |
| Euchlanis parva Rousselet, 1892 | Kou |
| Filinia cornuta (Weisse, 1848) | Ism |
| Filinia longiseta (Ehrenberg, 1834) | Amv, CCh, Chi, Doi, Kar, Kas, Ker, Kor, Lad, LAU, Lys, MgP, MkP, Oze, Pam, Par, Pet, Pin, Smo, Str, Tav, Tri, Veg, Vis, Vol, Vou, Yli, Zaz |
| Filinia opoliensis (Zacharias, 1898) | Lys, Oze, Tri |
| Filinia terminalis (Plate, 1886) | Amv, Ism, Lys, Pam, Smo, Tri, Veg, Vou |
| Gastropus hyptopus (Ehrenberg, 1838) | MkP, Thi |
| Gastropus stylifer Imhof, 1891 | DFe, Kas, Kre, Lad, MgP, MkP, Pin, Str, Sty, Thi, Tri, Vol |
| Hexarthra bulgarica (Wiszniewski, 1933) | Kas, Kor |
| Hexarthra intermedia (Wiszniewski, 1929) | Oze, Tri |
| Hexarthra mira (Hudson, 1871) | Amv, Chi, Doi, Kas, Kor, Lys, MkP, Oze, Pam, Par, Pet, Str, Tri, Veg, Vis, Vol, Vou, Zaz |
| Hexarthra oxyure (Zernov, 1903) | Kou, Vou |
| Hexarthra polyodonta (Hauer, 1957) | Kor |
| Kellicottia longispina (Kellicott, 1879) | Ali, Amv, Kas, Ker, LAU, MgP, MkP, Oze, Pam, Smo, Tav, Thi, Tri, Veg, Vol, Vou |
| Keratella cochlearis (Gosse, 1851) | Ali, Amv, CCh, Chi, DFe, Doi, Ism, Kar, Kas, Ker, KNe, Kor, Kre, Lad, LAU, Lys, MgP, MkP, Oze, Pam, Par, Pet, Pik, Smo, Str, Tav, Thi, Tri, Veg, Vol, Vou, Yli, Zaz |
| Keratella quadrata (Müller, 1786) | Ali, Amv, CCh, Chi, Doi, Ism, Kar, Kas, Ker, KNe, Kor, Kou, LAU, Lys, MgP, MkP, Oze, Pam, Par, Pet, Pik, Smo, Sty, Tav, Thi, Tri, Veg, Vis, Vol, Vou, Yli, Zaz |
| Keratella tecta (Gosse, 1851) | Ali, Amv, CCh, Chi, Doi, Ism, Kar, Kas, Ker, KNe, Kor, Lad, LAU, Lys, MgP, MkP, Oze, Pam, Par, Pet, Smo, Tav, Thi, Tri, Veg, Vol, Vou, Yli, Zaz |
| Keratella tropica (Apstein, 1907) | Amv, Doi, Kar, Kas, Kor, Kre, Lys, Oze, Pam, Par, Tri, Veg, Vis, Vou, Yli |
| Lecane arcula Harring, 1914 | Kas, KNe, LAU |
| Lecane bifurca (Bryce, 1892) | LAU |
| Lecane bulla (Gosse, 1851) | Ali, Amv, CCh, Kas, KNe, Kor, Kre, LAU, MgP, MkP, Oze, Par, Veg, Vol, Vou |
| Lecane cf. nana (Murray, 1913) | LAU |
| Lecane closterocerca (Schmarda, 1859) | Ali, Chi, Ism, Kar, Kas, KNe, Kor, LAU, Veg, Vol, Vou, Zaz |
| Lecane curvicornis (Murray, 1913) | Kas |
| Lecane elsa Hauer, 1931 | CCh, KNe, Lys, MgP, MkP, Vol |
| Lecane flexilis (Gosse, 1886) | Kas, LAU, Pet |
| Lecane furcata (Murray, 1913) | CCh, Kas, KNe, Kor, MkP, Vou |
| Lecane hamata (Stokes, 1896) | Kas, KNe, Kor, LAU, Vou |
| Lecane inopinata Harring & Myers, 1926 | Vou |
| Lecane lamellata (Daday, 1893) | Kor |
| Lecane ludwigii (Eckstein, 1883) | Kas |
| Lecane luna (Müller, 1776) | Ali, Amv, CCh, Kas, KNe, Kor, Kou, LAU, Lys, MkP, Oze, Sty, Veg, Vol, Vou, Zaz |
| Lecane lunaris (Ehrenberg, 1832) | Ali, CCh, Chi, Kas, KNe, Kor, MgP, MkP, Sty, Veg, Zaz |
| Lecane mira (Murray, 1913) | MkP |
| Lecane niothis Harring & Myers, 1926 | LAU |
| Lecane quadridentata (Ehrenberg, 1830) | Ali, CCh, Ism, Kas, KNe, Kor, MgP, Str, Tri |
| Lecane spinulifera Edmondson, 1935 | MkP |
| Lecane stenroosi (Meissner, 1908) | LAU |
| Lecane stichaea group | KNe, LAU |
| Lecane subtilis Harring & Myers, 1926 | Kar, LAU |
| Lecane subulata (Harring & Myers, 1926) | Kou |
| Lecane ungulata (Gosse, 1887) | Oze |
| Lepadella acuminata (Ehrenberg, 1834) | Kas, KNe, Kre, LAU, Sty |
| Lepadella ehrenbergii (Perty, 1850) | CCh, MkP, Vou |
| Lepadella glossa Wulfert, 1960 | CCh |
| Lepadella ovalis (Müller, 1786) | Ali, KNe, MkP |
| Lepadella patella (Müller, 1773) | CCh, Kar, Kas, KNe, Kor, Kou, LAU, MgP, MkP, Pik |
| Lindia torulosa Dujardin, 1841 | Ali |
| Lophocharis salpina (Ehrenberg, 1834) | Kor, LAU |
| Macrochaetus altamirai (Arévalo, 1918) | CCh |
| Macrochaetus collinsii (Gosse, 1867) | Kou |
| Monommata actices Myers, 1930 | CCh, MkP |
| Mytilina bisulcata (Lucks, 1912) | Ali, KNe, LAU |
| Mytilina mucronata (Müller, 1773) | Ali, Zaz |
| Mytilina ventralis (Ehrenberg, 1830) | Kas, Kor, Thi |
| Notholca acuminata (Ehrenberg, 1832) | Ali, Chi, Doi |
| Notholca foliacea (Ehrenberg, 1838) | Ali |
| Notholca salina Focke, 1961 | Kor, Kou, Pik |
| Notholca squamula (Müller, 1786) | Ali, Kas, Lad, MgP, MkP, Par, Pet, Veg, Vol |
| Notholca striata (Müller, 1786) | Doi, Ism, Kar |
| Notommata pseudocerberus Beauchamp, 1908 | Ali |
| Plationus patulus (Müller, 1786) | Amv, Kas, Vou |
| Platyias quadricornis (Ehrenberg, 1832) | Ali, Kas, MkP, Oze, Pam, Veg, Vol, Vou |
| Pleurotrocha petromyzon Ehrenberg, 1830 | Ali |
| Ploesoma hudsoni (Imhof, 1891) | Ali, Kre, Oze, Str |
| Ploesoma truncatum (Levander, 1894) | Amv, Kas, Kre, Lys, MgP, Oze, Pet, Str, Tri |
| Polyarthra dolichoptera Idelson, 1925 | Ali, Chi, Doi, Kar, LAU, Lys, Oze, Pam, Tav, Tri, Veg, Vol, Vou |
| Polyarthra euryptera Wierzejski, 1891 | Doi, Kas, Kor, Par, Pet, Veg, Vol |
| Polyarthra luminosa Kutikova,1962 | Amv, Kas, Kre, Par, Tri, Veg |
| Polyarthra major Burckhardt, 1900 | Ali, Amv, Kas, Ker, Kor, Kou, Pet, Pin, Tri, Veg, Vol, Vou |
| Polyarthra minor Voigt, 1904 | Doi, Kas, MkP, Vol, Vou |
| Polyarthra remata Skorikov, 1896 | Amv, Kor, LAU, Smo, Sty, Tri, Vol, Vou |
| Polyarthra vulgaris Carlin, 1943 | Ali, Amv, CCh, Doi, Kas, Kre, Kor, Kou, Lad, Lys, MgP, MkP, Oze, Pam, Str, Tav, Tri, Veg, Vol, Vou, Zaz |
| Pompholyx complanata Gosse, 1851 | Amv, Kas, Kor, Par, Pet, Smo, Veg, Vol |
| Pompholyx sulcata Hudson, 1885 | Doi, CCh, Kar, Kas, Kor, LAU, MgP, MkP, Par, Pet, Tav, Thi, Tri, Veg, Vol, Yli, Zaz |
| Proales theodora (Gosse, 1887) | Ali |
| Proalides subtilis Rodewald, 1940 | Ism, Kar, LAU, Vou, Zaz |
| Proalides tentaculatus Beauchamp, 1907 | Kor |
| Resticula melandoca (Gosse, 1887) | Vou |
| Scaridium longicauda (Müller, 1786) | Ali, CCh, Kas, Lad, MkP |
| Squatinella lamellaris (Müller, 1786) | CCh, Kas, Kne, LAU, MgP, MkP |
| Squatinella rostrum (Schmarda, 1846) | Zaz |
| Synchaeta kitina Rousselet, 1902 | Vou |
| Synchaeta littoralis Rousselet, 1902 | Ali |
| Synchaeta monopus Plate, 1889 | LAU |
| Synchaeta oblonga Ehrenberg, 1832 | Ali, LAU, Vol, Vou |
| Synchaeta pectinata Ehrenberg, 1832 | Ali, Amv, Doi, Kas, MkP, Pet, Pin, Tav, Vol, Yli |
| Synchaeta stylata Wierzejski, 1893 | CCh, Kas, Lad, Lys, MgP, MkP, Oze, Thi, Tri, Veg |
| Synchaeta tremula (Muller,1786) | LAU |
| Testudinella aspis Carlin, 1939 | Kas |
| Testudinella patina (Hermann, 1783) | Ali, Kas, Kor, Oze |
| Testudinella truncata (Gosse, 1886) | Kou, Lad, Sty |
| Trichocerca bicristata (Gosse, 1887) | LAU, Sty |
| Trichocerca capucina (Wierzejski & Zacharias, 1893) | Amv, CCh, Chi, Doi, Kas, Ker, Kor, Kre, LAU, MgP, MkP, Pam, Par, Pet, Tav, Thi, Tri, Veg, Vol, Yli, Zaz |
| Trichocerca cylindrica (Imhof, 1891) | CCh, Chi, Kas, Ker, Kor, LAU, MgP, MkP, Pam, Pet, Smo, Thi, Zaz |
| Trichocerca dixonnuttalli (Jennings, 1903) | Pam, Vou |
| Trichocerca elongata (Gosse, 1886) | Ali, Kas, KNe, MkP |
| Trichocerca longiseta (Schrank, 1802) | Ali |
| Trichocerca porcellus (Gosse, 1851) | Kou, Thi |
| Trichocerca pusilla (Jennings, 1903) | Ali, CCh, Chi, Doi, Kas, KNe, Kor, LAU, MgP, MkP, Par, Pet, Veg, Vol, Vou |
| Trichocerca rattus (Müller, 1776) | Ali, Kas, Lad, MkP, Tri, Veg |
| Trichocerca ruttneri Donner, 1953 | Lys, Oze |
| Trichocerca similis (Wierzejski, 1893) | Amv, CCh, Chi, Doi, Kar, Kas, Kor, Kre, Lys, MgP, MkP, Oze, Pam, Smo, Str, Tav, Thi, Tri, Veg, Vol, Vou, Yli, Zaz |
| Trichocerca stylata (Gosse, 1851) | Kas, Kor, MgP, MkP, Pam, Par, Pet, Vol, Vou |
| Trichocerca tenuior (Gosse, 1886) | Lad |
| Trichocerca tigris (Müller, 1786) | Kas, Lad |
| Trichocerca weberi (Jennings, 1903) | Kas, MkP, Pet, Veg |
| Trichotria pocillum (Müller, 1776) | CCh, Kas, Lad, Sty, Vou |
| Trichotria tetractis (Ehrenberg, 1830) | Ali, KNe, Lad, LAU, Vou |
| Tripleuchlanis plicata (Levander, 1894) | Kor |
| Superorder | Order | Family | Genus | No Species |
|---|---|---|---|---|
| Gnesiotrocha | Collothecacea | Collothecidae | Collotheca | 2 |
| Flosculariacea | Conochilidae | Conochilus | 3 | |
| Hexarthridae | Hexarthra | 5 | ||
| Testudinellidae | Pompholyx | 2 | ||
| Testudinella | 3 | |||
| Trochosphaeridae | Filinia | 4 | ||
| Pseudotrocha | Ploima | Asplanchnidae | Asplanchna | 4 |
| Brachionidae | Anuraeopsis | 1 | ||
| Brachionus | 20 | |||
| Kellicottia | 1 | |||
| Keratella | 4 | |||
| Notholca | 5 | |||
| Plationus | 1 | |||
| Platyias | 1 | |||
| Dicranophoridae | Dicranophorus | 4 | ||
| Encentrum | 2 | |||
| Epiphanidae | Epiphanes | 3 | ||
| Euchlanidae | Euchlanis | 4 | ||
| Tripleuchlanis | 1 | |||
| Gastropodidae | Ascomorpha | 3 | ||
| Gastropus | 2 | |||
| Lecanidae | Lecane | 24 | ||
| Lepadellidae | Squatinella | 2 | ||
| Colurella | 6 | |||
| Lepadella | 5 | |||
| Lindidae | Lindia | 1 | ||
| Mytilinidae | Lophocharis | 1 | ||
| Mytilina | 3 | |||
| Notommatidae | Cephalodella | 9 | ||
| Eosphora | 2 | |||
| Eothinia | 1 | |||
| Monommata | 1 | |||
| Notommata | 1 | |||
| Pleurotrocha | 1 | |||
| Resticula | 1 | |||
| Proalidae | Proales | 1 | ||
| Proalides | 2 | |||
| Scaridiidae | Scaridium | 1 | ||
| Synchaetidae | Ploesoma | 2 | ||
| Polyarthra | 7 | |||
| Synchaeta | 7 | |||
| Trichocercidae | Trichocerca | 15 | ||
| Trichotriidae | Macrochaetus | 2 | ||
| Trichotria | 2 |
| Taxa | Where |
|---|---|
| Cephalodella spp. | Ali, Kas, KNe, LAU, Zaz |
| Collotheca spp. | Amv, CCh, Chi, Doi, Kas, Kre, Kor, Kou, Lad, LAU, MgP, MkP, Pam, Par, Pin, Smo, Thi, Tri, Vol, Vou, Zaz |
| Colurella sp. | Mpo |
| Conochilus sp. | DFe, Lad, Lys, Oze, Smo, Thi, Veg |
| Epiphanes sp. | Ali, Mpo |
| Euchlanis sp. | CCh, MgP, MkP, Oze, Sty, Veg, Zaz |
| Hexarthra sp. | Ism, Mpo, Smo |
| Lecane sp. | Kas, MkP, LAU |
| Lepadella sp. | Chi, Ism, Kas, KNe, MkP, Pet, Veg |
| Lindia sp. | LAU, Mpo |
| Monommata sp. | LAU, Smo, Tri |
| Notholca sp. | Chi, Kar, Pam |
| Pleurotrocha sp. | Ali |
| Polyarthra spp. | Chi, Doi, Ism, Kas, MgP, MkP, Par, Pet, Smo, Tav, Thi, Veg, Yli, Zaz |
| Proalides sp. | Chi, Kne |
| Ptygura sp. | Ali, Kas, KNe, LAU, Pik |
| Sinantherina sp. | Ali |
| Synchaeta spp. | Chi, Ism, Kas, Kor, Lad, MgP, Oze, Pam, Par, Pet, Pin, Smo, Str, Tav, Thi, Vou, Yli, Zaz |
| Testudinella sp. | Mpo |
| Trichocerca spp. | Ism, Pet, Pin, Smo, Vol, Vou |
| Trichotria sp. | MkP, Yli |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamou, G.; Savva, A.; Demertzioglou, M.; Michaloudi, E. Diversity of Rotifera (Subclass: Monogononta) from Inland Water Bodies in Greece: An Updated Checklist. Diversity 2022, 14, 451. https://doi.org/10.3390/d14060451
Stamou G, Savva A, Demertzioglou M, Michaloudi E. Diversity of Rotifera (Subclass: Monogononta) from Inland Water Bodies in Greece: An Updated Checklist. Diversity. 2022; 14(6):451. https://doi.org/10.3390/d14060451
Chicago/Turabian StyleStamou, Georgia, Agni Savva, Maria Demertzioglou, and Evangelia Michaloudi. 2022. "Diversity of Rotifera (Subclass: Monogononta) from Inland Water Bodies in Greece: An Updated Checklist" Diversity 14, no. 6: 451. https://doi.org/10.3390/d14060451