Prioritizing Areas for Primate Conservation in Argentina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
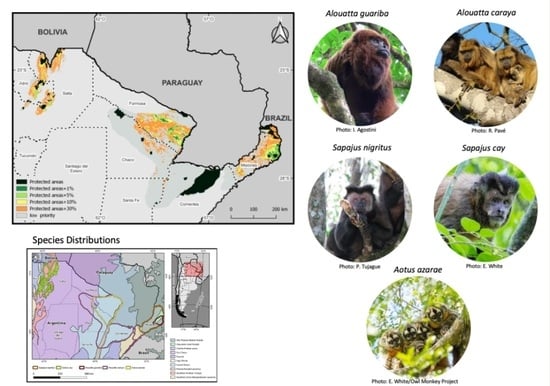
2.2. Study Species
2.3. Expert Knowledge
2.4. Species Distribution Models
2.5. Post-Processing Steps
2.6. Spatial Prioritization Analysis
2.6.1. Connectivity
2.6.2. Human Influence
2.6.3. Conservation Targets
2.6.4. Uncertainty Analysis
2.7. Comparisons of Anthropic Scenarios
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Species | Algorithm | Boyce | AUC | Jaccard | Sørensen | TSS |
|---|---|---|---|---|---|---|
| Alouatta guariba | MED | 0.81 | 0.86 | 0.68 | 0.81 | 0.59 |
| MES | 0.86 | 0.88 | 0.7 | 0.82 | 0.61 | |
| SVM | 0.85 | 0.87 | 0.7 | 0.82 | 0.62 | |
| RF | 0.93 | 0.92 | 0.73 | 0.84 | 0.68 | |
| PCS * | 0.93 | 0.92 | 0.73 | 0.84 | 0.68 | |
| Alouatta caraya | MED | 0.91 | 0.84 | 0.65 | 0.79 | 0.57 |
| MES | 0.89 | 0.82 | 0.62 | 0.76 | 0.53 | |
| SVM | 0.95 | 0.85 | 0.63 | 0.77 | 0.57 | |
| RF | 0.91 | 0.89 | 0.7 | 0.83 | 0.66 | |
| PCS * | 0.91 | 0.89 | 0.7 | 0.83 | 0.66 | |
| Aotus azarae | MED | 0.55 | 0.85 | 0.72 | 0.84 | 0.66 |
| MES | 0.7 | 0.84 | 0.72 | 0.83 | 0.65 | |
| SVM | 0.48 | 0.83 | 0.69 | 0.81 | 0.63 | |
| RF | 0.72 | 0.87 | 0.73 | 0.84 | 0.69 | |
| PCS * | 0.74 | 0.87 | 0.72 | 0.84 | 0.65 | |
| Sapajus nigritus | MED | 0.77 | 0.86 | 0.71 | 0.83 | 0.61 |
| MES | 0.71 | 0.87 | 0.72 | 0.84 | 0.63 | |
| SVM | 0.59 | 0.82 | 0.7 | 0.82 | 0.6 | |
| RF | 0.92 | 0.86 | 0.71 | 0.83 | 0.62 | |
| PCS * | 0.89 | 0.88 | 0.72 | 0.84 | 0.63 | |
| Sapajus cay | MED | 0.19 | 0.32 | 0.43 | 0.6 | 0.06 |
| MES | −0.08 | 0.3 | 0.28 | 0.37 | 0.11 | |
| SVM * | 0.67 | 0.53 | 0.46 | 0.6 | 0.35 | |
| RF | 0.11 | 0.47 | 0.41 | 0.54 | 0.27 | |
| PCS | 0.25 | 0.49 | 0.43 | 0.56 | 0.3 |
References
- SAyDS. SAREM Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción. Lista Roja de los Mamíferos de Argentina. 2019. Available online: https://cma.sarem.org.ar/ (accessed on 1 March 2020).
- Abba, A.M.; Varela, D.; Cirignoli, S.; Pereira, J.A.; Bolkovic, M.L.; Peker, S.; Porini, G.M.; de Bustos, S.; Degrati, M.; Denuncio, P.; et al. Categorización de los mamíferos de Argentina 2019: Resumen y análisis de las amenazas. Mastozoología Neotrop. 2022, 29, 1–73. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Nanni, A.S.; Piquer-Rodríguez, M.; Rodríguez, D.; Regueiro, M.N.; Periago, M.E.; Aguiar, S.; Ballari, S.; Blundo, C.; Derlindati, E.; Di Blanco, Y.; et al. Presiones sobre la conservación asociadas al uso de la tierra en las ecorregiones terrestres de la Argentina. Ecol. Austral 2020, 30, 304–320. [Google Scholar] [CrossRef]
- UMSEF. Monitoreo de la Superficie de Bosque Nativo de la República Argentina; Tomo, I., Ed.; Unidad de Manejo del Sistema de Evaluación Forestal, Dirección Nacional de Bosques, Ministerio de Ambiente y Desarrollo Sostenible de la Nación: Buenos Aires, Argentina, 2021. [Google Scholar]
- Holzmann, I.; Agostini, I.; Areta, J.I.; Ferreyra, H.; Beldomenico, P.; Di Bitetti, M.S. Impact of yellow fever outbreaks on two howler monkey species (Alouatta guariba clamitans and A. caraya) in Misiones, Argentina. Am. J. Primatol. 2010, 72, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.J.; Wich, S.A. Why conserve primates? In An Introduction to Primate Conservation; Wich, S.A., Marshall, A.J., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 13–31. [Google Scholar]
- Bello, C.; Galetti, M.; Pizo, M.A.; Magnago, L.F.S.; Rocha, M.F.; Lima, R.A.F.; Peres, C.A.; Ovaskainen, O.; Jordano, P. Defaunation affects carbon storage in tropical forests. Sci. Adv. 2015, 1, e1501105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, C.A.; Emilio, T.; Schietti, J.; Desmoulière, S.J.M.; Levi, T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl. Acad. Sci. USA 2016, 113, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar]
- Kukkala, A.; Moilanen, A. Core concepts of spatial prioritisation in systematic conservation planning. Biol. Rev. 2012, 88, 443–464. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Pressey, R.L.; Faith, D.P.; Margules, C.R.; Fuller, T.; Stoms, D.M.; Moffett, A.; Wilson, K.A.; Williams, K.J.; Williams, P.H.; et al. Biodiversity Conservation Planning Tools: Present Status and Challenges for the Future. Annu. Rev. Environ. Resour. 2006, 31, 123–159. [Google Scholar] [CrossRef]
- Lehtomäki, J.; Moilanen, A. Methods and workflow for spatial conservation prioritization using Zonation. Environ. Model. Softw. 2013, 47, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Rezende, G.; Sobral-Souza, T.; Culot, L. Integrating climate and landscape models to prioritize areas and conservation strategies for an endangered arboreal primate. Am. J. Primatol. 2020, 82, e23202. [Google Scholar] [CrossRef]
- Condro, A.; Prasetyo, L.; Rushayati, S.; Santikayasa, I.; Iskandar, E. Predicting Hotspots and Prioritizing Protected Areas for Endangered Primate Species in Indonesia under Changing Climate. Biology 2021, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.R.; Nowak, K.; Perkin, A. Priority Primate Areas in Tanzania. Oryx 2013, 48, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Calle-Rendón, B.R.; de Toledo, J.J.; Mustin, K.; Vasconcelos, I.M.; Hilário, R.R. Priority areas for conservation of primates in a threatened Amazonian savanna. J. Nat. Conserv. 2021, 65, 126109. [Google Scholar] [CrossRef]
- Tobón, W.; Urquiza-Haas, T.; Ramos-Fernández, G.; Calixto-Pérez, E.; Alarcón, J.; Kolb, M.; Koleff, P. Prioridades Para la Conservación de Los Primates en México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad–Asociación Mexicana de Primatología, A.C.–Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2012. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A New Global Map of Terrestrial Ecoregions Provides an Innovative Tool for Conserving Biodiversity. Bioscience 2001, 51, 933–938 . [Google Scholar] [CrossRef]
- Di Bitetti, M.S.; Janson, C.H. Reproductive socioecology of tufted capuchins (Cebus apella nigritus) in Norteastern Argentina. Int. J. Primatol. 2001, 22, 127–142. [Google Scholar]
- Di Bitetti, M.S.; Placci, G.; Dietz, L.A. A Biodiversity Vision for the Upper Paraná Atlantic Forest Ecoregion: Designing a Biodiversity Conservation Landscape and Setting Priorities for Conservation Action; World Wildlife Fund: Washington, DC, USA, 2003. [Google Scholar]
- Neiff, J.J.; Neiff, M. Evaluación de los impactos del cambio climático sobre el ecosistema natural y la biodiversidad Esteros del Iberá (Argentina). Ser. Medio Ambient. y Desarro. Santiago Chile Nac. Unidas. 2013, 152, 1–58. Available online: https://repositorio.cepal.org/bitstream/handle/11362/35885/LCL3728_es.pdf?sequence=1&isAllowed=y (accessed on 1 March 2020).
- Sampedro, D.; Calvi, M. Caracterización de la ganadería vacuna del nordeste argentino (NEA). In Cría Vacuna En el NEA; Barbera, P., Ed.; Ediciones INTA: Buenos Aires, Argentina, 2018; pp. 8–18. [Google Scholar]
- Orozco, M.M.; Marull, C.; Jiménez, I.; Gürtler, R.E. Mortalidad invernal de ciervo de los pantanos (Blastocerus dichotomus) en humedales del noreste argentino. Mastozoología Neotrop. 2013, 20, 163–170. [Google Scholar]
- Pizzio, R.; Bendersky, D. Base forrajera y potencial de mejora en el NEA. In Cría vacuna en el NEA; Barbera, P., Ed.; Ediciones INTA: Buenos Aires, Argentina, 2018; pp. 19–44. [Google Scholar]
- Brown, A.; Martinez Ortiz, U.; Acerbi, M.; Corcuera, J. La Situación Ambiental Argentina 2005; Fundación Vida Silvestre Argentina: Buenos Aires, Argentina, 2006. [Google Scholar]
- Bianchi, A.R.; Cravero, S.A.C. Atlas climático digital de la República Argentina; Instituto Nacional del Tecnología Agropecuaria (INTA): Salta, Argentina, 2010. [Google Scholar]
- Ginzburg, R.; Adámoli, J. Situación ambiental en el Chaco Húmedo. In La Situación Ambiental Argentina 2005; Brown, A., Martinez Ortiz, U., Acerbi, M., Corchera, J., Eds.; Fundación Vida Silvestre Argentina: Buenos Aires, Argentina, 2005; pp. 103–113. [Google Scholar]
- Fernandez-Duque, E. Social monogamy in wild owl monkeys (Aotus azarae) of Argentina: The potential influences of resource distribution and ranging patterns. Am. J. Primatol. 2015, 78, 355–371. [Google Scholar] [CrossRef] [Green Version]
- Portillo-Quintero, C.; Sánchez-Azofeifa, G. Extent and conservation of tropical dry forests in the Americas. Biol. Conserv. 2010, 143, 144–155. [Google Scholar] [CrossRef]
- Ojeda, R.; Stadler, J.; Brandl, R. Diversity of mammals in the tropical–temperate Neotropics: Hotspots on a regional scale. Biodivers. Conserv. 2003, 12, 1431–1444. [Google Scholar] [CrossRef]
- Burkart, R.; Bárbaro, N.O.; Sánchez, R.O.; Gómez, D.A. Ecoregiones de la Argentina; Administración de Parque Nacionales, Programa Desarrollo Institucional Ambiental: Buenos Aires, Argentina, 1999. [Google Scholar]
- Oklander, L.I.; Kowalewski, M.M.; Peker, S.M.; Pavé, R.; Agostini, I.; Holzmann, I.; Apellaniz, M. Alouatta caraya. In Categorización del Estado de Conservación de Los Mamíferos de Argentina 2019; Lista Roja de los mamíferos de Argentina; SaydS, S., Ed.; SAREM: Buenos Aires, Argentina, 2019; Available online: http://cma.sarem.org.ar/es/especie-nativa/alouatta-caraya (accessed on 1 March 2020).
- Brown, A.D.; Pacheco, S.; Lomáscolo, T.; Malizia, L. Situación ambiental en los bosques andinos yungueños. In La Situación Ambiental Argentina 2005; Brown, A.D., Martinez Ortiz, U., Acerbi, M., Corcuera, J., Eds.; Fundación Vida Silvestre Argentina: Buenos Aires, Argentina, 2005; pp. 53–61. [Google Scholar]
- Minetti, J.L.; Bobba, M.E.; Hernández, C. Régimen espacial de temperaturas en el Noroeste de Argentina. In El Clima del Noroeste Argentino; Minetti, J.L., Ed.; Laboratorio Climatológico Sudamericano (LCS), Editorial Magna: Tucuman, Argentina, 2012; pp. 141–161. [Google Scholar]
- Morello, J.; Matteucci, S.D.; Rodríguez, A.F.; Silva, M. Ecorregiones y Complejos Ecosistémicos Argentinos, 1st ed.; Orientacion Gráfica Editora: Buenos Aires, Argentina, 2012. [Google Scholar]
- Kinzey, W.G. Distribution of primates and forest refuges. In Biological diversification in the Tropics; Prance, G.T., Ed.; Columbia University Press: New York, NY, USA, 1982; pp. 455–482. [Google Scholar]
- Agostini, I.; Holzmann, I.; Oklander, L.I.; Peker, S.M.; Pavé, R.; Kowalewski, M.M. Alouatta guariba. In Categorización del Estado de Conservación de Los Mamíferos de Argentina 2019; Lista Roja de Los Mamíferos de Argentina; SaydS, S., Ed.; SAREM: Buenos Aires, Argentina, 2019; Available online: http://cma.sarem.org.ar/es/especie-nativa/alouatta-guariba (accessed on 1 March 2020).
- Agostini, I.; Holzmann, I.; Di Bitetti, M.S. The brown howler monkey (Alouatta guariba clamitans) in Argentina: Current status, threats and conservation challenges. In Primatology in Argentina; Kowalewski, M.M., Oklander, L.I., Eds.; SAREM Series A—Mammalogical Research; SAREM: Buenos Aires, Argentina, 2017; Volume 2, pp. 235–254. ISBN 9789879849774. [Google Scholar]
- Oklander, L.I.; Buss, G.; Biccamarques, J.C.; Hirano, Z.B.; Chaves, Ó.M.; Assis Jardim, M.M.; Valença-Montenegro, M.M.; Mendes, S.L.; Neves, L.G.; Kowalewski, M.; et al. Brown howler monkey Alouatta guariba (Humboldt, 1812). In Primates in Peril: The World’s 25 Most Endangered Primates 2022–2023; Mittermeier, R.A., Reuter, K.E., Rylands, A.B., Jerusalinsky, L., Schwitzer, C., Strier, K.B., Ratszimbazafy, J., Humle, T., Eds.; IUCN SSC Primate Specialist Group, International Primatological Society, Re:wild: Washington, DC, USA, 2022; pp. 121–125. [Google Scholar]
- Jerusalinsky, L.; Bicca-Marques, J.C.; Neves, L.G.; Alves, S.L.; Ingberman, B.; Buss, G.; Fries, B.G.; Alonso, A.C.; da Cunha, R.G.T.; Miranda, J.M.D.; et al. Alouatta guariba (Brown Howler Monkey)-(amended version of 2020 assessment). In The IUCN Red List of Threatened Species 2021; International Union for Conservation of Nature and Natural Resources: Glenn, Switzerland, 2021; p. e.T39916A190417874. [Google Scholar]
- Agostini, I.; Holzmann, I.; Di Bitetti, M.S. Ranging patterns of two syntopic howler monkey species (Alouatta guariba and A. caraya) in Northeastern Argentina. Int. J. Primatol. 2010, 31, 363–381. [Google Scholar] [CrossRef]
- Agostini, I.; Pizzio, E.; De Angelo, C.; Di Bitetti, M.S. Population status of primates in the Atlantic Forest of Argentina. Int. J. Primatol. 2015, 36, 244–258. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C.; Rumiz, D.I.; Ludwig, G.; Rímoli, J.; Martins, V.; da Cunha, R.G.T.; Alves, S.L.; Valle, R.R.; Miranda, J.M.D.; Jerusalinsky, L.; et al. Alouatta caraya (Black-and-gold howler monkey)-(amended version of 2020 assessment). In The IUCN Red List of Threatened Species 2021; International Union for Conservation of Nature and Natural Resources: Glenn, Switzerland, 2021; p. e.T41545A190414715. [Google Scholar]
- Kowalewski, M.M.; Pavé, R.; Fernández, V.A.; Raño, M.; Zunino, G.E. Life-history traits and group dynamic in black and gold howler monkeys in flooded forests of northern Argentina. In Primates in Flooded Habitats: Ecology and Conservation; Nowak, K., Barnett, A., Matsuda, I., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 263–269. ISBN 9781107134317. [Google Scholar]
- Juárez, C.P.; Dvoskin, R.; Fernandez-Duque, E. Structure and composition of wild black howler troops (Alouatta caraya) in gallery forests of the Argentinean Chaco. Neotrop. Primates 2005, 13, 19–22. [Google Scholar] [CrossRef]
- Fernandez-Duque, E.; Juárez, C.P.; Defler, T. Distribution, systematics and taxonomy. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernandez-Duque, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Fernandez-Duque, E.; Rotundo, M.; Sloan, C. Density and population structure of owl monkeys (Aotus azarai) in the Argentinean chaco. Am. J. Primatol. 2001, 53, 99–108. [Google Scholar] [CrossRef]
- Rímoli, J.; Pinto, T.; Romero-Valenzuela, D.; Rumiz, D.I.; Lynch Alfaro, J.W.; Ravetta, A.L. Aotus azarae (Azara’s night monkey) -(amended version of 2018 assessment). In The IUCN Red List of Threatened Species 2021; International Union for Conservation of Nature and Natural Resources: Glenn, Switzerland, 2021; p. e.T41539A190450485. [Google Scholar]
- Briggs, E.; Rotundo, M.; Dávalos, V.; García de la Chica, A.; Fernández-Duque, E. Aotus azarae. In Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción; Lista Roja de los Mamíferos de Argentina; Sayds, S., Ed.; SAREM: Buenos Aires, Argentina, 2019; Available online: http://cma.sarem.org.ar/es/especie-nativa/aotus-azarae (accessed on 1 March 2020).
- Fernandez-Duque, E.; Huck, M.; Van Belle, S.; Di Fiore, A. The evolution of pair-living, sexual monogamy, and cooperative infant care: Insights from research on wild owl monkeys, titis, sakis, and tamarins. Am. J. Phys. Anthr. 2020, 171, 118–173. [Google Scholar] [CrossRef]
- Zunino, G.E.; Galliari, C.A.; Colillas, O.J. Distribución y conservación del mirikiná (Aotus azarae) en Argentina: Resultados preliminares. In A Primatologia no Brasil 2; De Mello, M.T., Ed.; Sociedade Brasileira dePrimatologia: Brasília, Brazil, 1985; pp. 305–316. [Google Scholar]
- Tujague, M.P.; Agostini, I.; Oklander, L.; Peker, S.; Pfoh, R.; Baldovino, M.C.; Nieves, M.; Apellaniz, M. Sapajus nigritus. In Categorización del Estado de Conservación de los Mamíferos de Argentina 2019; Lista Roja de los Mamíferos de Argentina; SaydS, S., Ed.; SAREM: Buenos Aires, Argentina, 2019; Available online: http://cma.sarem.org.ar/es/especie-nativa/sapajus-nigritus (accessed on 1 March 2020).
- Ludwig, G.; de Melo, F.R.; Martins, W.P.; Miranda, J.M.D.; Lynch Alfaro, J.W.; Alonso, A.C.; dos Santos, M.C.; Rímoli, J. Sapajus nigritus (Black-horned capuchin)-(amended version of 2021 assessment). In The IUCN Red List of Threatened Species 2022; International Union for Conservation of Nature and Natural Resources: Glenn, Switzerland, 2022; p. e.T136717A210336199. [Google Scholar]
- Di Bitetti, M.; Kowalewski, M.; Lynch Alfaro, J.; Rylands, A.B. Sapajus nigritus ssp. cucullatus (Southern black-horned capuchin). In The IUCN Red List of Threatened Species 2020; International Union for Conservation of Nature and Natural Resources: Glenn, Switzerland, 2020; p. e.T160945956A160945959. [Google Scholar]
- Janson, C.; Baldovino, M.C.; Di Bitetti, M. The group life cycle and demography of brown capuchin monkeys (Cebus [apella] nigritus) in Iguazú National Park, Argentina. In Long-Term Field Studies of Primates; International Union for Conservation of Nature and Natural Resources; Kappeler, P.M., Watts, D.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 185–212. ISBN 9783642225130. [Google Scholar]
- Di Bitetti, M.S. Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. J. Zool. 2001, 253, 33–45. [Google Scholar] [CrossRef]
- Rímoli, J.; Smith, L.; Ludwig, G.; Martinez, M.; Kowalewski, M.; Melo, R.; Lynch, W. Sapajus cay (Azara’s capuchin). In The IUCN Red List of Threatened Species 2022; International Union for Conservation of Nature and Natural Resources: Glenn, Switzerland, 2022; Volume 35, p. e.T136366A215548623. [Google Scholar]
- Nieves, M.; Peker, S.; Tujague, M.P.; Kowalewski, M.; Pavé, R.; Agostini, I.; Alcalde, A.S.; Albanesi, S.; de Bustos, S.; Jayat, J.P.; et al. Sapajus cay. In Categorización del Estado de Conservación de los Mamíferos de Argentina 2019; Lista Roja de los Mamíferos de Argentina; SaydS, S., Ed.; SAREM: Buenos Aires, Argentina, 2019; Available online: http://cma.sarem.org.ar/es/especie-nativa/sapajus-cay (accessed on 1 March 2020).
- Brown, A.D.; Zunino, G.E. Dietary variability in Cebus apella in extreme habitats: Evidence for adaptability. Folia Primatol. 1990, 54, 187–195. [Google Scholar] [CrossRef]
- Calixto-Pérez, E.; Alarcón-Guerrero, J.; Ramos-Fernández, G.; Dias, P.A.D.; Rangel-Negrín, A.; Améndola-Pimenta, M.; Domingo, C.; Arroyo-Rodríguez, V.; Pozo-Montuy, G.; Pinacho-Guendulain, B.; et al. Integrating expert knowledge and ecological niche models to estimate Mexican primates’ distribution. Primates 2018, 59, 451–467. [Google Scholar] [CrossRef]
- Kareksela, S.; Aapala, K.; Alanen, A.; Haapalehto, T.; Kotiaho, J.; Lehtomäki, J.; Leikola, N.; Mikkonen, N.; Moilanen, A.; Nieminen, E.; et al. Combining spatial prioritization and expert knowledge facilitates effectiveness of large-scale mire protection process in Finland. Biol. Conserv. 2019, 241, 108324. [Google Scholar] [CrossRef]
- Sánchez-Fernández, D.; Lobo, J.M.; Hernández-Manrique, O.L. Species distribution models that do not incorporate global data misrepresent potential distributions: A case study using Iberian diving beetles. Divers. Distrib. 2010, 17, 163–171. [Google Scholar] [CrossRef]
- Culot, L.; Pereira, L.A.; Agostini, I.; de Almeida, M.A.B.; Alves, R.S.C.; Aximoff, I.; Bager, A.; Baldovino, M.C.; Bella, T.R.; Bicca-Marques, J.C.; et al. ATLANTIC-PRIMATES: A dataset of communities and occurrences of primates in the Atlantic Forests of South America. Ecology 2018, 100, e02525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mccarthy, K.P.; Jr., R.J.F.; Rota, C.T.; Hutto, R.L. Predicting species distributions from samples collected along roadsides. Conserv. Biol. 2011, 26, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Varela, S.; Anderson, R.P.; García-Valdés, R.; Fernández González, F. Environmental filters reduce the effects of sampling bias and improve predictions of ecological niche models. Ecography 2014, 37, 1084–1091. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [Green Version]
- De Marco, P.; Nóbrega, C.C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef]
- Messager, M.L.; Lehner, B.; Grill, G.; Nedeva, I.; Schmitt, O. Estimating the volume and age of water stored in global lakes using a geo-statistical approach. Nat. Commun. 2016, 7, 13603. [Google Scholar] [CrossRef]
- Lehner, B.; Grill, G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 2013, 27, 2171–2186. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Soberón, J.; Peterson, A.T. No silver bullets in correlative ecological niche modelling: Insights from testing among many potential algorithms for niche estimation. Methods Ecol. Evol. 2015, 6, 1126–1136. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Karatzoglou, A.; Smola, A.; Hornik, K.; Zeileis, A. kernlab- AnS4Package for Kernel Methods inR. J. Stat. Softw. 2004, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence-absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Zhu, G.-P.; Peterson, A.T. Do consensus models outperform individual models? Transferability evaluations of diverse modeling approaches for an invasive moth. Biol. Invasions 2017, 19, 2519–2532. [Google Scholar] [CrossRef]
- De Andrade, A.F.A.; Velazco, S.J.E.; Júnior, P.D.M. ENMTML: An R package for a straightforward construction of complex ecological niche models. Environ. Model. Softw. 2020, 125, 104615. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Velazco, S.J.E.; Ribeiro, B.R.; Laureto, L.M.O.; Júnior, P.D.M. Overprediction of species distribution models in conservation planning: A still neglected issue with strong effects. Biol. Conserv. 2020, 252, 108822. [Google Scholar] [CrossRef]
- Fortes, V.B.; Bicca-Marques, J.C.; Urbani, B.; Fernández, V.A.; Pereira, T.d.S. Ranging behavior and spatial cognition of howler monkeys. In Howler Monkeys, Developments in Primatology: Progress and Prospects; Kowalewski, M., Garber, P., Cortés-Ortiz, L., Urbani, B., Youlatos, D., Eds.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 219–255. [Google Scholar]
- Wartmann, F.M.; Juárez, C.P.; Fernandez-Duque, E. Size, site fidelity, and overlap of home ranges and core areas in the socially monogamous owl monkey (Aotus azarae) of Northern Argentina. Int. J. Primatol. 2014, 35, 919–939. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, A.; Pouzols, F.M.; Meller, L.; Veach, V.; Arponen, A.; Leppänen, J.; Kujala, H. Zonation Version 4 User Manual; C-BIG Conservation Biology Informatics Group, Department of Biosciences, University of Helsinki: Helsinki, Finland, 2014. [Google Scholar]
- Moilanen, A.; Anderson, B.J.; Eigenbrod, F.; Heinemeyer, A.; Roy, D.B.; Gillings, S.; Armsworth, P.R.; Gaston, K.J.; Thomas, C.D. Balancing alternative land uses in conservation prioritization. Ecol. Appl. 2011, 21, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, A.; Franco, A.M.A.; Early, R.I.; Fox, R.; Wintle, B.; Thomas, C.D.; Moilanen, A.; Franco, A.M.A.; Early, R.I.; Fox, R.; et al. Prioritizing multiple-use landscapes for conservation: Methods for large multi-species planning problems. Proc. R. Soc. B: Boil. Sci. 2005, 272, 1885–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouzols, F.M.; Moilanen, A. A method for building corridors in spatial conservation prioritization. Landsc. Ecol. 2014, 29, 789–801. [Google Scholar] [CrossRef]
- Zunino, G.E.; Kowalewski, M.M.; Oklander, L.I.; González, V. Habitat fragmentation and population size of the black and gold howler monkey (Alouatta caraya) in a semideciduous forest in Northern Argentina. Am. J. Primatol. 2007, 69, 966–975. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.G.; Ribeiro, M.C.; Hasui, E.; da Costa, C.A.; da Cunha, R.G.T. Patch size, functional isolation, visibility and matrix permeability influences neotropical primate occurrence within highly fragmented landscapes. PLoS ONE 2015, 10, e0114025. [Google Scholar] [CrossRef] [Green Version]
- Duque, F. Natal dispersal in monogamous owl monkeys (Aotus azarai) of the Argentinean Chaco. Behaviour 2009, 146, 583–606. [Google Scholar] [CrossRef]
- Raño, M.; Kowalewski, M.M.; Cerezo, A.M.; Garber, P.A. Determinants of daily path length in black and gold howler monkeys (Alouatta caraya) in northeastern Argentina. Am. J. Primatol. 2016, 78, 825–837. [Google Scholar] [CrossRef]
- Mikkonen, N.; Moilanen, A. Identification of top priority areas and management landscapes from a national Natura 2000 network. Environ. Sci. Policy 2013, 27, 11–20. [Google Scholar] [CrossRef] [Green Version]
- CBD First Draft of the Post-2020 Global Biodiversity Framework CBD/WG2020/3/3. 5 July 2021. Available online: https://www.cbd.int/doc/c/abb5/591f/2e46096d3f0330b08ce87a45/wg2020-03-03-en.pdf (accessed on 19 October 2022).
- Moilanen, A.; Wintle, B.A.; Elith, J.; Burgman, M. Uncertainty Analysis for Regional-Scale Reserve Selection. Conserv. Biol. 2006, 20, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Di Bitetti, M.S. Primates bark-stripping trees in forest plantations—A review. For. Ecol. Manag. 2019, 449, 117482. [Google Scholar] [CrossRef]
- Cowling, R.; Pressey, R.; Sims-Castley, R.; le Roux, A.; Baard, E.; Burgers, C.; Palmer, G. The expert or the algorithm?—Comparison of priority conservation areas in the Cape Floristic Region identified by park managers and reserve selection software. Biol. Conserv. 2003, 112, 147–167. [Google Scholar] [CrossRef]
- Fernández-Duque, F.; Huck, M.; Dávalos, V.; Fernández-Duque, E. Estudio preliminar sobre la ecología, el comportamiento y la demografía del muitú (Crax fasciolata) en la selva en galería del Riacho Pilagá, Formosa, Argentina. Hornero 2013, 28, 65–74. [Google Scholar]
- Smichowski, H.; del Rosario Montiel, M.; Romero, V.; Kowalewski, M.; Contreras, F.I. Evaluación de incendios en áreas periurbanas de la ciudad de Corrientes (Argentina) durante el año 2020. Papeles Geogr. 2021, 67, 151–167. [Google Scholar] [CrossRef]
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; Di Fiore, A.; Nekaris, K.A.-I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Murillo, F.; Arguedas, R. Blood analytes of electrocuted mantled howler monkeys (Alouatta palliata) in the Nicoya peninsula of Costa Rica. J. Med. Primatol. 2021, 50, 231–239. [Google Scholar] [CrossRef]
- Pozo-Montuy, G.; Bonilla-Sánchez, Y.M. Population decline of an endangered primate resulting from the impact of a road in the Catazajá wetlands, Chiapas, México. Therya Notes 2022, 3, 75–81. [Google Scholar] [CrossRef]
- Tomas, W.M.; Berlinck, C.N.; Chiaravalloti, R.M.; Faggioni, G.P.; Strüssmann, C.; Libonati, R.; Abrahão, C.R.; Alvarenga, G.D.V.; Bacellar, A.E.D.F.; Batista, F.R.D.Q.; et al. Distance sampling surveys reveal 17 million vertebrates directly killed by the 2020’s wildfires in the Pantanal, Brazil. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Di Bitetti, M.S.; Mata, J.; Svenning, J.-C. Exotic mammals and rewilding in the Neotropics. Mastozoología Neotrop. 2022, 29, 001–015. [Google Scholar] [CrossRef]
- Di Bitetti, M.S.; De Angelo, C.; Paviolo, A.; Di Giacomo, A.S.; Varela, D.; Giraudo, A.R. Argentina: Wildfires jeopardize rewilding. Nature 2022, 604, 246. [Google Scholar] [CrossRef] [PubMed]
- Agostini, I.; Holzmann, I.; Di Bitetti, M.S.; Oklander, L.I.; Kowalewski, M.M.; Beldomnico, P.M.; Goenaga, S.; Martínez, M.; Moreno, E.S.; Lestani, E.; et al. Building a species conservation strategy for the brown howler monkey (Alouatta guariba clamitans) in Argentina in the context of yellow fever outbreaks. Trop. Conserv. Sci. 2014, 7, 25–34. [Google Scholar] [CrossRef]
- Moreno, E.S.; Agostini, I.; Holzmann, I.; Di Bitetti, M.S.; Oklander, L.I.; Kowalewski, M.M.; Beldomenico, P.M.; Goenaga, S.; Martínez, M.; Lestani, E.; et al. Yellow fever impact on brown howler monkeys (Alouatta guariba clamitans) in Argentina: A metamodelling approach based on population viability analysis and epidemiological dynamics. Mem. Inst. Oswaldo Cruz 2015, 110, 865–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.M. Primate crop feeding behavior, crop protection, and conservation. Int. J. Primatol. 2017, 38, 385–400. [Google Scholar] [CrossRef]
- Spagnoletti, N.; Cardoso, T.C.M.; Fragaszy, D.; Izar, P. Coexistence between humans and capuchins (Sapajus libidinosus): Comparing observational data with farmers’ perceptions of crop losses. Int. J. Primatol. 2016, 38, 243–262. [Google Scholar] [CrossRef]
- Corley, M.; Fernandez-Duque, E. Dispersal: A critical life-history stage influencing populations, social dynamics, and individual fitness. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernandez-Duque, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Gorostiaga, N.; Zamboni, L.P.; Cerezo, A.; Pavé, R. Influencia de los atributos del paisaje en la ocupación de parches de bosque y en la demografía de Alouatta caraya en un ambiente fragmentado del noreste de Argentina. Rev. Mex. Biodivers. 2021, 92, 923475. [Google Scholar] [CrossRef]
- Howard, C.; Stephens, P.A.; Pearce-Higgins, J.W.; Gregory, R.D.; Willis, S.G. Improving species distribution models: The value of data on abundance. Methods Ecol. Evol. 2014, 5, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Sans, G.H.C.; Aguiar, S.; Vallejos, M.; Paruelo, J.M. Assessing the effectiveness of a land zoning policy in the Dry Chaco. The case of Santiago del Estero, Argentina. Land Use Policy 2017, 70, 313–321. [Google Scholar] [CrossRef]



| National | Distribution (km2) | PA | PA + 1% | PA + 5% | PA + 10% | PA + 30% | |
|---|---|---|---|---|---|---|---|
| HIS | A. guariba | 4962 | 12.5% | 24.3% | 58.2% | 80.0% | 99.7% |
| A. caraya | 164957 | 7.7% | 10.3% | 17.8% | 26.1% | 54.7% | |
| A. azarae | 49905 | 1.3% | 5.9% | 17.4% | 29.3% | 67.5% | |
| S. nigritus | 28189 | 14.7% | 17.4% | 25.6% | 34.5% | 68.0% | |
| S. cay | 23611 | 14.0% | 14.0% | 23.3% | 34.6% | 80.4% | |
| US | A. guariba | 4962 | 12.5% | 30.1% | 75.4% | 96.1% | 99.9% |
| A. caraya | 164957 | 7.7% | 10.4% | 18.2% | 25.8% | 53.7% | |
| A. azarae | 49905 | 1.3% | 5.3% | 15.8% | 25.9% | 66.3% | |
| S. nigritus | 28189 | 14.7% | 18.6% | 30.3% | 42.1% | 78.8% | |
| S. cay | 23611 | 14.0% | 14.0% | 23.1% | 39.0% | 89.1% | |
| Atlantic Forest | |||||||
| HIS | A. guariba | 4962 | 13.2% | 19.9% | 46.5% | 71.6% | 97.9% |
| A. caraya | 27077 | 12.5% | 13.8% | 19.3% | 25.5% | 47.5% | |
| S. nigritus | 26918 | 15.1% | 16.7% | 23.0% | 30.5% | 56.0% | |
| US | A. guariba | 4962 | 16.3% | 24.3% | 52.2% | 79.8% | 99.5% |
| A. caraya | 27077 | 12.7% | 14.1% | 19.6% | 25.5% | 48.4% | |
| S. nigritus | 26918 | 15.5% | 17.3% | 23.9% | 30.9% | 56.8% | |
| Chaco | |||||||
| HIS | A. caraya | 137880 | 5.3% | 8.1% | 18.1% | 28.7% | 63.5% |
| A. azarae | 49905 | 1.6% | 5.6% | 17.6% | 31.9% | 79.5% | |
| US | A. caraya | 137880 | 5.3% | 8.0% | 17.5% | 27.9% | 63.3% |
| A. azarae | 49905 | 1.6% | 6.1% | 21.7% | 39.0% | 85.1% | |
| Yungas | |||||||
| HIS | S. cay | 23611 | 15.6% | 16.7% | 21.3% | 27.0% | 50.5% |
| US | S. cay | 23611 | 16.7% | 18.0% | 23.4% | 30.0% | 55.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostini, I.; Velazco, S.J.E.; Insaurralde, J.A.; Pavé, R.; Holzmann, I.; Fernández-Duque, E.; Tujague, M.P.; Peker, S.; Kowalewski, M.M.; Di Bitetti, M.S. Prioritizing Areas for Primate Conservation in Argentina. Diversity 2022, 14, 982. https://doi.org/10.3390/d14110982
Agostini I, Velazco SJE, Insaurralde JA, Pavé R, Holzmann I, Fernández-Duque E, Tujague MP, Peker S, Kowalewski MM, Di Bitetti MS. Prioritizing Areas for Primate Conservation in Argentina. Diversity. 2022; 14(11):982. https://doi.org/10.3390/d14110982
Chicago/Turabian StyleAgostini, Ilaria, Santiago José Elías Velazco, Juan Ariel Insaurralde, Romina Pavé, Ingrid Holzmann, Eduardo Fernández-Duque, María Paula Tujague, Silvana Peker, Martín M. Kowalewski, and Mario Santiago Di Bitetti. 2022. "Prioritizing Areas for Primate Conservation in Argentina" Diversity 14, no. 11: 982. https://doi.org/10.3390/d14110982