1. Introduction
Climate change in relation to temperature and precipitation, as well as variation in snow cover, atmospheric nitrogen deposition, and dispersal, are the main recognized factors that affect plant species distribution in alpine environments [
1]. Globally, alpine regions are expected to experience more warming than other regions, while at the same time, experiencing increased human pressure from tourism and land-use changes. These cumulative impacts threaten alpine species, mainly plants, and habitats [
2]. The Global Observational Research Initiative in Alpine Environments (GLORIA, University of Natural Resources and Life Sciences, Austria) is an international science program with collaborators in over 130 locations worldwide, to monitor the effects of climate change on vegetation, temperature, and other variables over time (e.g., [
3]). Soil temperature is considered more critical than atmospheric conditions for alpine plants due to the close relationship between soil and underground roots and meristems [
4]. Soil temperature and moisture are affected by topography, which alters snow distribution, incident radiation, wind exposure, and soil properties, which determine the zonation of some plant communities (e.g., [
5,
6,
7]).
Most studies developed within the GLORIA framework focus on Europe and the northern hemisphere (e.g., [
8,
9,
10,
11]), where global warming has forced many alpine species to move upward mountains, modifying plant composition at specific locations. This phenomenon has been corroborated in temperate, boreal, subtropical, and tropical ecosystems (e.g., [
9,
12,
13,
14]). Recently, the GLORIA-Andes group, working with data from seven South American countries, reported interesting results from the southern hemisphere but under continental conditions (e.g., [
15,
16,
17]) that also demonstrated the thermophilization of plant species composition [
18]. These results, however, did not include information about South American sites at high latitudes (greater than 45° SL), such as South Patagonia. Studies on the effect of climate change on alpine biodiversity at high latitudes are still scarce in the southern hemisphere, under oceanic conditions [
19], as well as in different landscapes, since typical vegetation surrounding mountains are usually forests but rarely grasslands. Furthermore, the regional climate in the southern hemisphere is strongly influenced by other natural climatic events or phenomena, including the El Niño Southern Oscillation (ENSO) [
20] and the Antarctic Circumpolar Current (ACC) [
21]. These events strongly influence past and present climate at high latitudes (e.g., [
22]), but their influence on high elevation temperatures has been rarely explored [
17].
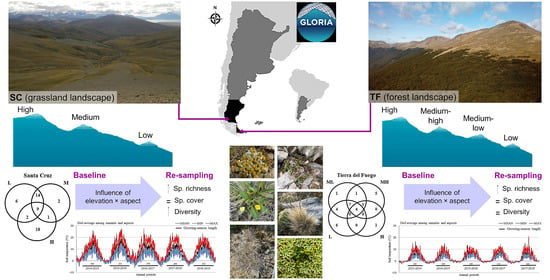
South Patagonia is an Argentinean region that extends from 46° to 56° SL and from 63° to 73° WL, which includes two provinces: Santa Cruz (SC), at the southern extreme of the South American continent, and Tierra del Fuego (TF), an archipelago separated from the continent by the Magellan Strait. The landscape of this region is home to several semi-natural habitats, including arid grasslands dominated by
Festuca and
Stipa species, and deciduous and evergreen forests, dominated by
Nothofagus species, but also peatlands and scrublands [
23]. Patagonian grasslands have been grazed by domestic livestock (mainly sheep) for over 100 years [
24], mainly under continuous grazing in large and heterogeneous paddocks [
25].
Nothofagus forests have been used since colonization for timber production, and they currently sustain recreational/touristic activities [
26]. Alpine environments have not been intensively and productively used in South Patagonia, but livestock breeding in SC and tourist activity in TF have increased in recent times in the mountain areas. Previous studies of southern Patagonian alpine environments have been mostly related to taxonomy (e.g., [
27,
28,
29,
30,
31]) and plant distribution according to topographic or geomorphological factors [
32,
33]. However, there is a lack of information about temporal changes in alpine plants and temperatures at high southern latitudes. Therefore, the objectives of this work were: (1) to evaluate variations in alpine vascular plant diversity between the baseline and first re-sampling after a five-year period, at different topographic (elevations and cardinal aspects) conditions, in mountains located in two contrasting landscapes (foothill grasslands and sub-Antarctic forests) of South Patagonia; (ii) to analyze main variations in soil temperatures and growing-season length over five annual periods following the baseline sampling, relating them to ENSO climatic variations. We hypothesized that (i) alpine plant composition varies with dominant vegetation type of the surrounding landscape (forest or grassland), and richness, cover and diversity indices diminish with elevation gain, expecting even lower values in more exposed cardinal aspects, while changes in time mirror general trends caused by global warming (movement upward of thermophilic plant species, and loss of cryophilic species); (ii) soil temperatures (mean, minimum and maximum) and growing-season length diminish with elevation gain, expecting even lower values in more exposed cardinal aspects, and increase over time because of global warming, although ENSO climatic variations also impact on these variables. At these southern latitudes, the large deficiency of floristic alpine vegetation studies and the lack of evidence about topographical and temporal changes in alpine vegetation and soil temperature dynamics from long-term studies, reinforce the importance of this study.
4. Discussion
The analyzed plant species assemblages, both in SC and TF, were mostly composed of typical lowland species (more than 70% of the total richness in each site) that are naturally widely distributed across a wide range of elevations. For example,
Azorella monantha,
Chiliotrichum diffusum, and
Nassauvia darwinii, occupy broad latitudinal (e.g., from 32° to 54° LS) and altitudinal gradients (e.g., from 0 to 3000 m a.s.l.) [
39]. However, these species are smaller in alpine habitats compared with lowlands, as was observed in
Chiliotrichum diffusum (>1 m average height in lowlands [
29], but <20 cm average height in alpine habitats). On the other hand, only a small proportion (17% of total richness in SC and 10% in TF) were mountain species. The presence of lowland species at high elevations is common poleward, due to similarities between environmental conditions at high elevations and high latitudes, but this has been much more described for arctic regions [
3]. On the other hand, several of the observed species (e.g.,
Adesmia aphanantha,
Astragalus palenae,
Tristagma nivale, and
V. moyanoi) are naturally widely distributed across mountains of the entire Patagonia [
39]. However, we only found them in one province (SC or TF); this is likely because plant communities in mountains are locally more heterogeneous and patchier than in lowlands, as these communities can only survive in restricted favorable microsites when the climate becomes less hospitable at higher elevations [
47] or latitudes. In summary, assemblages were different between the mountain sites of SC and TF, without any species in common, reflecting differences in the surrounding vegetation and partially confirming our first hypothesis. Differences in species composition between the two sites were also highlighted by exclusive and endemic species, some of which were also identified as indicator species of the studied assemblages, mainly in Tierra del Fuego. The high representation of endemism among the indicator species has also been observed in mountains in other parts of the world (e.g., [
10]). However, the lack of knowledge about the vascular alpine plant assemblage composition in other mountainous regions of SC and TF makes it difficult to extrapolate endemic species value as indicator species for the entire region, even at similar elevations and aspects. More studies are needed across mountains to improve knowledge about species specificity and fidelity to a given elevation and aspect in South Patagonia. For example, through this study, 5 species in SC (
Acaena platyacantha,
Benthamiella spegazziniana,
Philippiella patagonica,
V. moyanoi, and
V. magellanica) and 3 in TF (
A. antarctica,
S. alloeophyllus var.
alloeophyllus, and
S. humifusus) were found outside of their expected elevation ranges, as was reported in other GLORIA works [
17].
The presence of exotic species in alpine plant assemblages is usually uncommon [
48,
49], although
P. pratensis was also observed in high mountain areas of México [
50]. In our study, the presence of exotic species may be related to domestic (e.g., sheep, cows, and horses) and/or native (
L. guanicoe) herbivorous mammals that graze freely in the study area, as is often observed in other alpine ecosystems (e.g., [
51]). The spread of exotic plants could be facilitated by the increase in accessibility (e.g., [
52]). However, the disappearance of
D. glomerata in the re-sampling of SC denoted their low capability to survive in alpine conditions. Despite observed differences in the two studied sites, some taxonomic overlapping occurred between them when the species list of the entire summit area (up to 10 m down a level from the top) was compared. For example,
Azorella lycopodioides,
B. microphylla, and
M. grandiflorum were common species in both provinces across the entire summit area (data non-shown), displaying the influence of the sampling method.
The increase in richness and diversity comparing re-sampling to baseline in 1 m
2 quadrats, as well as the upward movement of species, both in SC and TF, is consistent with the short-term rise in species richness in other monitoring studies (e.g., [
19,
53,
54,
55]). This could be interpreted as an early indicator of climate change-driven warming, despite we did not detect soil warming over the studied five-year period. Also, downslope range shifts may constitute an indirect response to warming caused by changes in species interactions, as well as to habitat modification [
56]. Instead of long temporal temperature data series in South Patagonia outside cities, climate change estimations following CSIRO model in B2 scenario predicted for 2080, +3 °C warming in the mean maximum temperature in SC and +2 °C in the north of TF [
37]. Drivers of changes in richness and movement of species should be confirmed with more specific studies, such as those performed by [
9,
18,
57], who reported upward mountain movements of more-thermophilic species and downslope shifts caused by warming [
56].
Monthly mean and absolute maximum and minimum temperatures did not increase over time in our study, with the highest values in the middle annual periods (2015–2016 and 2016–2017 for SC, 2016–2017 for TF). In the period 2016–2017, SC showed the longest growing season. Ref. [
19] also did not observe a gain in soil temperatures over a seven-year period at a GLORIA pilot site off the southern coast of Australia, but they did find an increase in species richness. These authors argue that the increase in species richness in those Australian alpine areas could be explained by the influence of other environmental factors (e.g., rainfall, microsite availability, species interactions, and dispersal/recruitment potential in the regional species pool) rather than climate warming. The influence of ENSO has already been demonstrated in South American alpine environments [
17]. We found contrasting effects of ENSO in soil temperature and growing season in the two sites of the present study, that found inverse trends (e.g., higher soil temperatures in SC, but lower soil temperatures in TF during a La Niña event). How ENSO affects mountain climate in South Patagonia should be studied in greater depth. Additionally, the observed increase in alpine species richness could be a delayed expression of vegetation changes [
58], which could start and be sustained by greater warming rates that occurred many years ago (e.g., during the 70s decade), as is commonly observed in lags in tree regeneration advances above established treelines [
59]. In this sense, long-term studies in remote places like Patagonia are important to better understand the effect of climate change on plant richness and vegetation cover in alpine areas across the southern hemisphere.
Elevation had an important influence on vegetation and soil temperature in SC and TF, as it was expected based on other mountain studies (e.g., [
8,
10], richness, cover, and mean temperatures generally decrease with elevation). In contrast to TF, the highest summit of SC contained the highest amount of exclusive species (
Figure 1). Species whose distribution was restricted to the highest summit have a particularly strong tolerance for high mountain extreme climatic conditions (e.g., [
60,
61]), more adaptations to the cold [
62], and low competition abilities [
63]. It is likely that the continuity of high Andean mountain peaks (>1000 m a.s.l.) favors the colonization of mountain areas in SC, from northern to southern latitudes, by species with particular requirements typically found in the mountains of the central Argentinean Andes (Río Negro and Mendoza provinces) up to 2000 m a.s.l. [
39]. Some of these species include
A. aphanantha,
Astragalus nivicola, and
Leucheria leontopodioides. In contrast, the continuity of high mountain peaks is interrupted to the south by a low, hilly area near the Magellan Strait, generating a division between the continent and the high mountain chains of TF (Cordillera Darwin). In addition to the change in direction of the Andes from north-south to west-east direction in TF, this division increases the distance to other alpine islands and disrupts alpine plant species dispersion, as has also been observed in the mountains of central Mexican [
50]. Many other species, mentioned as characteristic of and exclusive to high elevation mountains in SC and TF (e.g.,
Moschopis rosulata,
Baccharis nivalis) [
39], were not found in our study. This could be due to the restricted dispersal abilities of plants in alpine environments, where there is a high prevalence of barochory [
64]. However, other authors have suggested that species present in the upper limit of vegetation are controlled by the availability of safe sites for colonization, survival, and growth among rocky substrate [
47]. Concerning soil temperature, the snow cover on the higher summits was highly variable and likely depended on micro-topography (e.g., slopes on the steepest peaks are windswept and snow does not persist very long there even in the winter), as other alpine studies have reported (e.g., [
45,
65,
66,
67]).
At lower summits on both sites, plant species composition was highly influenced by the surrounding vegetation. Lowland species, with few specific requirements and a high tolerance for mountain temperatures and soil conditions, were able to expand their distribution to the lower summits at both studied landscapes. These species included
F. pallescens in grasslands and
A. ranunculus in forests. Other species inhabiting mountains were also common in lowland ecosystems (e.g., xeric steppes, peat bogs), species such as
A. prolifera,
V. magellanica and
Senecio neaei in SC, and
Austrolycopodium magellanicum and
Gaultheria pumila var.
pumila in TF. The species that occurred at all elevations (9 in SC and 4 in TF,
Figure 1 and
Appendix A), had broad habitat ranges, morphological variability, and latitudinal distributions (e.g.,
A. monantha,
Azorella selago,
Nardophyllum bryoides,
E. rubrum,
Luzula alopecurus), including several dwarf shrubs and cushions with adaptations to survive under different climatic conditions. Regarding soil temperatures, we registered a long series of values around 0 °C in the lowest summits on both sites, due to deep snow cover in wintertime (from May to October) that removes diurnal temperature variations (e.g., [
45,
65,
66,
67]).
The cardinal aspect also had a strong influence on vegetation assemblages and length of the growing season both in SC and in TF and was more important than the elevation factor in defining plant assemblages on some summits. This trend was also observed in European mountains by [
11,
55]. Particularly, northern and western aspects on the highest summit of SC contained only two species (
Oxalis loricata and
Nassauvia sp.), with very low cover for each (less than 0.1%). The high exposure of these aspects to the cold, dry, and strong (up to 200 km per h) glacial winds, a lack of well-developed soil on very steep slopes, and proximity to icefields (5 km closer than lower summits), reduced plant species establishment and the species pool able to tolerate these conditions. This wind-induced distribution pattern of vegetation in the alpine belt was also observed in the Himalayas [
68], where temperatures below 0 °C and the absence of snow protection restricts and limits plant establishment (e.g., on steep slopes).
On the other hand, the significantly different plant assemblages on eastern aspects of the low and medium elevation summits of SC are likely explained by better soil and hydrological conditions, allowing for the growth of species less tolerant to xeric environments (e.g.,
F. pallescens). Plant species assemblages on eastern aspects were more similar to those of surrounding lowland grasslands exposed to continuous grazing [
69]. In contrast, vegetation on north, south, and west-facing plots can be affected by soil erosion and limited water availability for plant growth. This could favor the establishment of prostrated shrubs with taproot systems (e.g.,
N. bryoides). Conversely, in deeper soils, erosion produces organic matter and nutrient loss in the superficial horizons, generating a sandy texture that favors psamophilic species (e.g.,
P. chrysophylla var.
chrysophylla) [
70]. On the other hand, northern slopes were warmer, with earlier snow-melt and longer growing season (
Appendix B), probably resulting in earlier vegetation sprouting, and an increased livestock preference for grazing and sleeping when compared to colder and snowier southern aspects [
69]. The favorable growing conditions produced by earlier snowmelt and higher summer temperatures on sunnier exposures (south-facing in the northern hemisphere) at low elevations have been documented previously in other alpine habitats across Europe [
65] and Asia [
45]. Likewise, the significant dominance of xerophilous species, as well as some woody dwarf shrubs (e.g.,
E. chilensis) and cushion plants (e.g.,
N. glomerulosa), shows that mountain species in grassland landscapes of South Patagonia are not only associated with temperature but also with water availability, as has been observed in other mountain ecosystems [
19,
71]. Pits and mounds, as well as rock outcroppings, may also influence soil moisture distribution and stocking. These relationships should be better studied in South Patagonia alpine environments.
In TF, aspect had a stronger influence on vegetation and soil temperature, particularly in high and medium-high elevation summits. Similar to the results obtained in SC, two aspects of the highest elevation summit in TF (southern and western) showed a clear different plant assemblage that had highly reduced richness and cover. The unique species that can survive there (e.g.,
S. alloeophyllus var.
alloeophyllus) are likely those adapted to live on rocks and screes [
8]. On the other hand, the species assemblages on north and east-facing plots at the high elevation summit, which were similar to those on northern, southern, and western facing plots at the medium-high summit, consisted of species adapted to receive abundant meltwater or to live in flooded areas (e.g.,
A. antarctica). Likewise, plant assemblages on the east aspect at medium-high summit exhibited similar composition and cover as those on lower summits. Different aspects on the lower summit showed little variation because of the flat topography, and similar exposure to conditioning factors. In addition, the highest maximum and the lowest minimum temperature in the northern aspect of the highest summit can be explained by the fact that these areas are always free of snow, even during the coldest months, because of their exposure to strong winds. These winds cause winter temperatures to drop very low, while during the growing season they become warmer than other aspects, due to strong insolation [
45]. Although temperatures in the favorable growth period are important, a thermal condition in the unfavorable period and days with freezing temperatures are significant as well [
66]. Additional studies are required to better understand temperature-related limitations in South Patagonia.