Serotiny in Primula palinuri: How to Face the Dry Season on Mediterranean Cliffs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites and Plant Materials
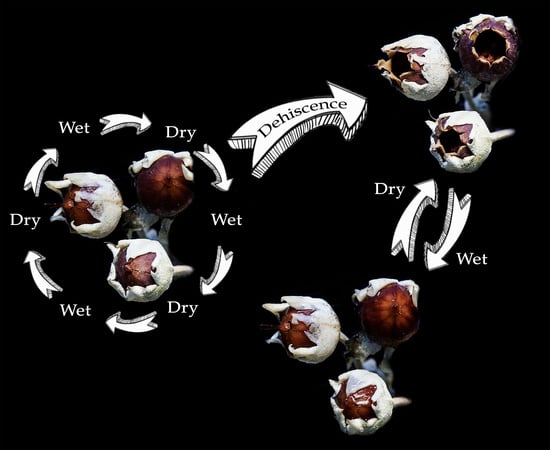
2.2. Capsule Dehiscence
2.3. Field Experiments: Seed Storage
2.3.1. Storage in Aerial Seed Bank
2.3.2. Storage in Soil Seed Bank
2.4. Statistical Analysis
2.4.1. Capsule Dehiscence and the Effect of Wax Removal
2.4.2. Seed Storage on Plants
3. Results
3.1. Capsule Dehiscence
3.2. Aerial and Soil Seed Bank
4. Discussion
4.1. Serotiny in P. palinuri
4.2. From What We Know to What Is Still Missing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hewitt, G. The genetic legacy of the quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2005; ISBN 0521019214. [Google Scholar]
- Davis, P.H. Cliff vegetation in the eastern mediterranean. J. Ecol. 1951, 39, 63–93. [Google Scholar] [CrossRef]
- Cooper, A. Plant species coexistence in cliff habitats. J. Biogeogr. 1997, 24, 483–494. [Google Scholar] [CrossRef]
- Aronne, G.; De Micco, V.; Santangelo, A.; Santo, A.; Buonanno, M. Coastal vertical cliffs of the National Park of Cilento: Reservoirs of endemic species. Latest Trends Eng. Mech. Struct. Eng. Geol. 2014, 26, 77–85. [Google Scholar]
- Lavergne, S.; Thompson, J.D.; Garnier, E.; Debussche, M. The biology and ecology of narrow endemic and widespread plants: A comparative study of trait variation in 20 congeneric pairs. Oikos 2004, 107, 505–518. [Google Scholar] [CrossRef]
- Richards, J. Primula; Pavilion Books: London, UK, 2014; ISBN 1849942412. [Google Scholar]
- Darwin, C. On the two Forms, or dimorphic condition, in the species of Primula, and on their remarkable sexual relations. J. Proc. Linn. Soc. London. Bot. 1862, 6, 77–96. [Google Scholar] [CrossRef]
- Ehrlén, J.; Käck, S.; Ågren, J. Pollen limitation, seed predation and scape length in Primula farinosa. Oikos 2002, 97, 45–51. [Google Scholar] [CrossRef]
- Antrobus, S.; Lack, A.J. Genetics of colonizing and established populations of Primula veris. Heredity 1993, 71, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, J.C. Primula Scotica hook. J. Ecol. 1954, 42, 623. [Google Scholar] [CrossRef]
- Davidson, J.B.; Wolf, P.G. Natural history of maguire primrose, Primula cusickiana var. Maguirei (Primulaceae). West. North Am. Nat. 2012, 71, 327–337. [Google Scholar] [CrossRef]
- Crema, S.; Cristofolini, G.; Rossi, M.; Conte, L. High genetic diversity detected in the endemic Primula apennina Widmer (Primulaceae) using ISSR fingerprinting. Plant Syst. Evol. 2009, 280, 29–36. [Google Scholar] [CrossRef]
- Jiménez, A.; Mansour, H.; Keller, B.; Conti, E. Low genetic diversity and high levels of inbreeding in the Sinai primrose (Primula boveana), a species on the brink of extinction. Plant Syst. Evol. 2014, 300, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.B.; Kadereit, J.W. Classification of Primula sect. Auricula (Primulaceae) based on two molecular data sets (ITS, AFLPs), morphology and geographical distribution. Bot. J. Linn. Soc. 2004, 146, 1–26. [Google Scholar]
- Aronne, G.; Arena, C.; De Micco, V.; Giovanetti, M.; Buonanno, M. Full light and soil drought constrain plant growth in Mediterranean cliffs: The case of Primula palinuri Petagna. Plant Biosyst. 2018, 152, 863–872. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Occurrence of morphological and anatomical adaptive traits in young and adult plants of the rare mediterranean cliff species Primula palinuri Petagna. Sci. World J. 2012, 2012, 471814. [Google Scholar] [CrossRef] [Green Version]
- Aronne, G.; Buonanno, M.; De Micco, V. Assessment of distyly syndrome in Primula palinuri Petagn. a rare species living on maritime vertical cliffs. Plant Syst. Evol. 2013, 300, 917–924. [Google Scholar] [CrossRef]
- Strumia, S.; Buonanno, M.; Aronne, G.; Santo, A.; Santangelo, A. Monitoring of plant species and communities on coastal cliffs: Is the use of unmanned aerial vehicles suitable? Diversity 2020, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Aronne, G.; De Micco, V.; Barbi, S. Hypocotyl features of Primula palinuri Petagna (Primulaceae), an endemic and rare species of the Southern Tyrrhenian Coast. In Proceedings of the Ecologia Emergenza Pianificazione, 18th Congresso Nazionale della Società Italiana di Ecologia (SItE ’10), Cagliari, Italy, 12–14 September 2010; Società Italiana di Ecologia: Parma, Italy, 2010; pp. 113–119. [Google Scholar]
- Aronne, G.; Buonanno, M.; De Micco, V. Reproducing under a warming climate: Long winter flowering and extended flower longevity in the only Mediterranean and maritime Primula. Plant Biol. 2014, 17, 535–544. [Google Scholar] [CrossRef]
- Aronne, G.; Wilcock, C. Reproductive phenology in Mediterranean macchia vegetation. Lagascalia 1997, 19, 445–454. [Google Scholar]
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecologica Oecologia Plant. 1980, 1, 245–252. [Google Scholar]
- Herrera, J. Flowering and fruiting phenology in the coastal shrublands of Doñana, south Spain. Vegetatio 1986, 68, 91–98. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier Science: San Diego, CA, USA, 2014; ISBN 0124166830. [Google Scholar]
- Van der Pijl, L. Principles of Dispersal in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1969; ISBN 978-3-642-96110-6. [Google Scholar]
- Lamont, B.B.; Enright, N.J. Adaptive advantages of aerial seed banks. Plant Species Biol. 2000, 15, 157–166. [Google Scholar] [CrossRef]
- Lamont, B.B.; Le Maitre, D.C.; Cowling, R.M.; Enright, N.J. Canopy seed storage in woody plants. Bot. Rev. 1991, 57, 277–317. [Google Scholar] [CrossRef]
- Gao, R.; Yang, X.; Yang, F.; Wei, L.; Huang, Z.; Walck, J.L. Aerial and soil seed banks enable populations of an annual species to cope with an unpredictable dune ecosystem. Ann. Bot. 2014, 114, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Lamont, B.B. Canopy seed storage and release: What’s in a name? Oikos 1991, 60, 266–278. [Google Scholar] [CrossRef]
- Saracino, A.; Bellino, A.; Allevato, E.; Mingo, A.; Conti, S.; Rossi, S.; Bonanomi, G.; Carputo, D.; Mazzoleni, S. Repeated stand-replacing crown fires affect seed morphology and germination in Aleppo pine. Front. Plant Sci. 2017, 8, 1160. [Google Scholar] [CrossRef] [Green Version]
- Bastida, F.; González-Andújar, J.L.; Monteagudo, F.J.; Menéndez, J. Aerial seed bank dynamics and seedling emergence patterns in two annual Mediterranean Asteraceae. J. Veg. Sci. 2010, 21, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Zedler, P.H. Closed-cone conifers of the chaparral. Fremontia 1986, 14, 14–17. [Google Scholar]
- Rossi, S.; Morin, H.; Gionest, F.; Laprise, D. Inter-and intra-annual patterns of seed rain in the black spruce stands of Quebec, Canada. IForest 2017, 10, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Berdeja, A.; Ezcurra, E.; Sanders, A.C. Delayed seed dispersal in California deserts. Madroño 2015, 62, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Bastida, F.; Talavera, S. Temporal and spatial patterns of seed dispersal in two Cistus species (Cistaceae). Ann. Bot. 2002, 89, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolin, P. Ombrohydrochory: Rain-operated seed dispersal in plants—with special regard to jet-action dispersal in Aizoaceae. Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 511–518. [Google Scholar] [CrossRef]
- Günster, A. Seed bank dynamics - longevity, viability and predation of seeds of serotinous plants in the central namib desert. J. Arid Environ. 1994, 28, 195–205. [Google Scholar] [CrossRef]
- Fox, G.A. Failure time analysis: Studying times-to-events and rates at which events occur. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: New Yor, NY, USA, 2001; pp. 253–289. ISBN 9780195131888. [Google Scholar]
- Lee, E.T.; Wang, J. Statistical Methods for Survival Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 9780471369974. [Google Scholar]
- R, Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wollenweber, E.; Schneider, H. Lipophilic exudates of pteridaceae—Chemistry and chemotaxonomy. Biochem. Syst. Ecol. 2000, 28, 751–777. [Google Scholar] [CrossRef]
- Strumia, S.; Croce, A.; Santangelo, A. New distributional data of the rare endemic species Eokochia saxicola (Guss.) Freitag and G. Kadereit (Chenopodiaceae): Effects on biogeography and conservation. Plant Biosyst. 2015, 149, 559–564. [Google Scholar] [CrossRef]
- Barone Lumaga, M.R.; Santangelo, A.; Strumia, S. Morpho-functional traits influencing the fitness of highly endangered Eokochia saxicola (Guss.) Freitag & G. Kadereit (Amaranthaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2016, 218, 11–17. [Google Scholar]
- Baker, E.A.; Hunt, G.M. Erosion of waxes from leaf surface by simulated rain. New Phytol. 1986, 102, 161–173. [Google Scholar] [CrossRef]
- Van Oudtshoorn, K.V.R.; Van Rooyen, M.W. Dispersal Biology of Desert Plants; Adaptations of Desert Organisms; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 3662035618. [Google Scholar]
- Lamont, B.B.; Groom, P.K. Seed and seedling biology of the woody-fruited proteaceae. Aust. J. Bot. 1998, 46, 387–406. [Google Scholar]
- Valverde, T.; Silvertown, J. An integrated model of demography, patch dynamics and seed dispersal in a woodland herb, Primula vulgaris. Oikos 1997, 80, 67–77. [Google Scholar] [CrossRef]
- Valverde, T.; Silvertown, J. Spatial variation in the seed ecology of a woodland herb (Primula vulgaris) in relation to light environment. Funct. Ecol. 1995, 9, 942–950. [Google Scholar] [CrossRef]
- MacMahon, J.A.; Mull, J.F.; Crist, T.O. Harvester ants (Pogonomyrmex spp.): Their community and ecosystem influences. Annu. Rev. Ecol. Syst. 2000, 31, 265–291. [Google Scholar] [CrossRef]
- Retana, J.; Picó, F.X.; Rodrigo, A. Dual role of harvesting ants as seed predators and dispersers of a non-myrmechorous Mediterranean perennial herb. Oikos 2004, 105, 377–385. [Google Scholar] [CrossRef]
- Leimu, R.; Syrjänen, K.; Ehrlén, J.; Lehtilä, K. Pre-dispersal seed predation in Primula veris: Among-population variation in damage intensity and selection on flower number. Oecologia 2002, 133, 510–516. [Google Scholar] [CrossRef] [PubMed]



| Variable | df | Parameter (SE) | χ2 | p-Value |
|---|---|---|---|---|
| Intercept | 1 | 2.46 (0.13) | 49.31 | <0.001 |
| Treatment | 1 | 19.67 | <0.001 | |
| (Wax removed) | 1 | −0.92 (0.18) | 19.67 | <0.001 |
| (Wax unremoved) | 0 | 0.00 | ||
| Scale parameter | 1 | 0.55 (0.11) | ||
| Maximized log-likelihood = −151.50 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, R.; Izzo, L.G.; Buonanno, M.; Aronne, G. Serotiny in Primula palinuri: How to Face the Dry Season on Mediterranean Cliffs. Diversity 2020, 12, 291. https://doi.org/10.3390/d12080291
Silvestro R, Izzo LG, Buonanno M, Aronne G. Serotiny in Primula palinuri: How to Face the Dry Season on Mediterranean Cliffs. Diversity. 2020; 12(8):291. https://doi.org/10.3390/d12080291
Chicago/Turabian StyleSilvestro, Roberto, Luigi Gennaro Izzo, Maurizio Buonanno, and Giovanna Aronne. 2020. "Serotiny in Primula palinuri: How to Face the Dry Season on Mediterranean Cliffs" Diversity 12, no. 8: 291. https://doi.org/10.3390/d12080291