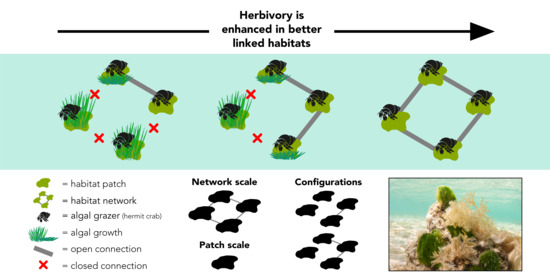
Being Well-Connected Pays in a Disturbed World: Enhanced Herbivory in Better-Linked Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Design
2.2. Press Versus Pulse Herbivory Experiments
2.3. Statistical Analyses
3. Results
3.1. Pulse Disturbance Experiment
3.2. Press Disturbance Experiment
3.3. Variability in Grazing Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Network Theory Metrics Used to Measure Configuration at the Patch and System Scale
Appendix A.2. Patch-Scale Configuration Metric
- In the original equation, pi refers to all other patches within the system; however, because we were only interested in the grazing power of the crabs coming from the undisturbed patches to each of the disturbed patches, our measure for patch configuration considers only the connectivity between the disturbed patch of interest and all undisturbed patches (Figure A1);
- Due to the limited number of patches and overall size of our system, closeness values were in the order of 1 × 10−3; therefore, we multiplied the original equation by 100 to obtain an easily interpretable value.

Appendix A.3. System-Scale Configuration Metric

References
- Beger, M.; Grantham, H.S.; Pressey, R.L.; Wilson, K.A.; Peterson, E.L.; Dorfman, D.; Mumby, P.J.; Lourival, R.; Brumbaugh, D.R.; Possingham, H.P. Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biol. Conserv. 2010, 143, 565–575. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T.; Norderhaug, K.M.; Ramirez-Llodra, E.; Pedersen, M.F. Movement of pulsed resource subsidies from kelp forests to deep fjords. Oecologia 2018, 187, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Adam, T.C.; Schmitt, R.J.; Holbrook, S.J.; Brooks, A.J.; Edmunds, P.J.; Carpenter, R.C.; Bernardi, G. Herbivory, connectivity, and ecosystem resilience: Response of a coral reef to a large-scale perturbation. PLoS ONE 2011, 6, e23717. [Google Scholar] [CrossRef] [PubMed]
- Magioli, M.; de Barros, K.M.P.M.; Setz, E.Z.F.; Percequillo, A.R.; Rondon, M.V.D.S.S.; Kuhnen, V.V.; Da Silva Canhoto, M.C.; Dos Santos, K.E.A.; Kanda, C.Z.; de Lima Fregonezi, G.; et al. Connectivity maintain mammal assemblages functional diversity within agricultural and fragmented landscapes. Eur. J. Wildl. Res. 2016, 62, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Westwood, A.R.; Lambert, J.D.; Reitsma, L.R.; Stralberg, D. Prioritizing areas for land conservation and forest management planning for the threatened Canada Warbler (Cardellina canadensis) in the Atlantic Northern Forest of Canada. Diversity 2020, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.G.E.; Bennett, E.M.; Gonzalez, A. Linking landscape connectivity and ecosystem service provision: Current knowledge and research gaps. Ecosystems 2013, 16, 894–908. [Google Scholar] [CrossRef]
- Noël, L.M.L.J.; Hawkins, S.J.; Jenkins, S.R.; Thompson, R.C. Grazing dynamics in intertidal rockpools: Connectivity of microhabitats. J. Exp. Mar. Bio. Ecol. 2009, 370, 9–17. [Google Scholar] [CrossRef]
- Olds, A.D.; Pitt, K.A.; Maxwell, P.S.; Connolly, R.M.; Frid, C. Synergistic effects of reserves and connectivity on ecological resilience. J. Appl. Ecol. 2012, 49, 1195–1203. [Google Scholar] [CrossRef]
- Ruiz-Montoya, L.; Lowe, R.J.; Kendrick, G.A. Contemporary connectivity is sustained by wind- and current-driven seed dispersal among seagrass meadows. Mov. Ecol. 2015, 3, 9. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Strydom, S.; Connolly, R.M.; Schoeman, D. Donor-control of scavenging food webs at the land-ocean interface. PLoS ONE 2013, 8, e68221. [Google Scholar] [CrossRef] [Green Version]
- Treml, E.A.; Ford, J.R.; Black, K.P.; Swearer, S.E. Identifying the key biophysical drivers, connectivity outcomes, and metapopulation consequences of larval dispersal in the sea. Mov. Ecol. 2015, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limberger, R.; Wickham, S.A. Predator dispersal determines the effect of connectivity on prey diversity. PLoS ONE 2011, 6, e29071. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, M.A.; Fletcher, R.J., Jr.; Tremblay, R.L.; Melendez-Ackerman, E.J. Spatial asymmetries in connectivity influence colonization-extinction dynamics. Oecologia 2015, 179, 415–424. [Google Scholar] [CrossRef]
- Elmhirst, T.; Connolly, S.R.; Hughes, T.P. Connectivity, regime shifts and the resilience of coral reefs. Coral Reefs 2009, 28, 949–957. [Google Scholar] [CrossRef]
- Hogan, J.D.; Thiessen, R.J.; Sale, P.F.; Heath, D.D. Local retention, dispersal and fluctuating connectivity among populations of a coral reef fish. Oecologia 2012, 168, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Elmqvist, T.; Folke, C.; Nystrom, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Mumby, P.J.; Elliott, I.A.; Eakin, C.M.; Skirving, W.; Paris, C.B.; Edwards, H.J.; Enriquez, S.; Iglesias-Prieto, R.; Cherubin, L.M.; Stevens, J.R. Reserve design for uncertain responses of coral reefs to climate change. Ecol. Lett. 2011, 14, 132–140. [Google Scholar] [CrossRef]
- Dikou, A. Ecological processes and contemporary coral reef management. Diversity 2010, 2, 717–737. [Google Scholar] [CrossRef]
- Vergés, A.; McCosker, E.; Mayer-Pinto, M.; Coleman, M.A.; Wernberg, T.; Ainsworth, T.; Steinberg, P.D.; Williams, G. Tropicalisation of temperate reefs: Implications for ecosystem functions and management actions. Funct. Ecol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Fox, R.J.; Bellwood, D.R. Quantifying herbivory across a coral reef depth gradient. Mar. Ecol. Prog. Ser. 2007, 339, 49–59. [Google Scholar] [CrossRef]
- Cheal, A.J.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral–macroalgal phase shifts or reef resilience: Links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar] [CrossRef]
- Poore, A.G.; Campbell, A.H.; Coleman, R.A.; Edgar, G.J.; Jormalainen, V.; Reynolds, P.L.; Sotka, E.E.; Stachowicz, J.J.; Taylor, R.B.; Vanderklift, M.A.; et al. Global patterns in the impact of marine herbivores on benthic primary producers. Ecol. Lett. 2012, 15, 912–922. [Google Scholar] [CrossRef]
- Ebrahim, A.; Olds, A.D.; Maxwell, P.S.; Pitt, K.A.; Burfeind, D.D.; Connolly, R.M. Herbivory in a subtropical seagrass ecosystem: Separating the functional role of different grazers. Mar. Ecol. Prog. Ser. 2014, 511, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, L.; Edwards, W.; York, P.H.; Rasheed, M.A. Richness of primary producers and consumer abundance mediate epiphyte loads in a tropical seagrass system. Diversity 2020, 12, 384. [Google Scholar] [CrossRef]
- Martin, T.S.H.; Olds, A.D.; Olalde, A.B.H.; Berkström, C.; Gilby, B.L.; Schlacher, T.A.; Butler, I.R.; Yabsley, N.A.; Zann, M.; Connolly, R.M. Habitat proximity exerts opposing effects on key ecological functions. Landsc. Ecol. 2018, 33, 1273–1286. [Google Scholar] [CrossRef] [Green Version]
- Engelhard, S.L.; Huijbers, C.M.; Stewart-Koster, B.; Olds, A.D.; Schlacher, T.A.; Connolly, R.M.; Österblom, H. Prioritising seascape connectivity in conservation using network analysis. J. Appl. Ecol. 2017, 54, 1130–1141. [Google Scholar] [CrossRef] [Green Version]
- Kindlmann, P.; Burel, F. Connectivity measures: A review. Landsc. Ecol. 2008, 23, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Driver, L.J.; Hoeinghaus, D.J. Fish metacommunity responses to experimental drought are determined by habitat heterogeneity and connectivity. Freshw. Biol. 2016, 61, 533–548. [Google Scholar] [CrossRef]
- Gilarranz, L.J.; Rayfield, B.; Linan-Cembrano, G.; Bascompte, J.; Gonzalez, A. Effects of network modularity on the spread of perturbation impact in experimental metapopulations. Science 2017, 357, 199–201. [Google Scholar] [CrossRef] [Green Version]
- Limberger, R.; Wickham, S.A. Transitory versus persistent effects of connectivity in environmentally homogeneous metacommunities. PLoS ONE 2012, 7, e44555. [Google Scholar] [CrossRef] [Green Version]
- Di Carvalho, J.A.; Wickham, S.A. Simulating eutrophication in a metacommunity landscape: An aquatic model ecosystem. Oecologia 2019, 189, 461–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, R.M.; Jinks, K.I.; Brown, C.J.; Schlacher, T.A.; Connolly, R.M. Functional changes in reef systems in warmer seas: Asymmetrical effects of altered grazing by a widespread crustacean mesograzer. Sci. Total Environ. 2018, 644, 976–981. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Skillington, A.J.; Connolly, R.M.; Robinson, W.; Gaston, T.F. Coupling between marine plankton and freshwater flow in the plumes off a small estuary. Int. Rev. Hydrobiol. 2008, 93, 641–658. [Google Scholar] [CrossRef]
- Wainger, L.; Yu, H.; Gazenski, K.; Boynton, W. The relative influence of local and regional environmental drivers of algal biomass (chlorophyll-a) varies by estuarine location. Estuar. Coast. Shelf Sci. 2016, 178, 65–76. [Google Scholar] [CrossRef]
- Yabsley, N.A.; Olds, A.D.; Connolly, R.M.; Martin, T.S.; Gilby, B.L.; Maxwell, P.S.; Huijbers, C.M.; Schoeman, D.S.; Schlacher, T.A. Resource type influences the effects of reserves and connectivity on ecological functions. J. Anim. Ecol. 2016, 85, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Netw. 1979, 1, 215–239. [Google Scholar] [CrossRef] [Green Version]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [Green Version]
- Saunders, M.I.; Brown, C.J.; Foley, M.M.; Febria, C.M.; Albright, R.; Mehling, M.G.; Kavanaugh, M.T.; Burfeind, D.D. Human impacts on connectivity in marine and freshwater ecosystems assessed using graph theory: A review. Mar. Freshw. Res. 2016, 67, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Tavella, J.; Cagnolo, L. Does fire disturbance affect ant community structure? Insights from spatial co-occurrence networks. Oecologia 2019. [Google Scholar] [CrossRef]
- Treml, E.A.; Halpin, P.N.; Urban, D.L.; Pratson, L.F. Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landsc. Ecol. 2007, 23, 19–36. [Google Scholar] [CrossRef]
- Beger, M.; Linke, S.; Watts, M.; Game, E.; Treml, E.; Ball, I.; Possingham, H.P. Incorporating asymmetric connectivity into spatial decision making for conservation. Conserv. Lett. 2010, 3, 359–368. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P. lme4: Linear Mixed-Effects Models Using ‘Eigen’ and S4; R package ver. 1.1-12; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Alexander, S.M. Snow-tracking and GIS: Using multiple species-environment models to determine optimal wildlife crossing sites and evaluate highway mitigation plans on the Trans-Canada Highway. Can. Geogr. 2008, 52, 169–187. [Google Scholar] [CrossRef]
- Nabe-Nielsen, J.; Sibly, R.M.; Forchhammer, M.C.; Forbes, V.E.; Topping, C.J. The effects of landscape modifications on the long-term persistence of animal populations. PLoS ONE 2010, 5, e8932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorenbosch, M.; Verberk, W.; Nagelkerken, I.; van der Velde, G. Influence of habitat configuration on connectivity between fish assemblages of Caribbean seagrass beds, mangroves and coral reefs. Mar. Ecol. Prog. Ser. 2007, 334, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Gilby, B.L.; Olds, A.D.; Connolly, R.M.; Henderson, C.J.; Schlacher, T.A. Spatial restoration ecology: Placing restoration in a landscape context. BioScience 2018, 68, 1007–1019. [Google Scholar] [CrossRef]
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Maxwell, P.S. Primacy of seascape connectivity effects in structuring coral reef fish assemblages. Mar. Ecol. Prog. Ser. 2012, 462, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Duncan, C.K.; Gilby, B.L.; Olds, A.D.; Connolly, R.M.; Ortodossi, N.L.; Henderson, C.J.; Schlacher, T.A. Landscape context modifies the rate and distribution of predation around habitat restoration sites. Biol. Conserv. 2019, 237, 97–104. [Google Scholar] [CrossRef]
- Kleyheeg, E.; van Dijk, J.G.B.; Tsopoglou-Gkina, D.; Woud, T.Y.; Boonstra, D.K.; Nolet, B.A.; Soons, M.B. Movement patterns of a keystone waterbird species are highly predictable from landscape configuration. Mov. Ecol. 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Bodin, Ö.; Tengö, M. Disentangling intangible social–ecological systems. Glob. Environ. Chang. 2012, 22, 430–439. [Google Scholar] [CrossRef]
- Crook, D.A.; Lowe, W.H.; Allendorf, F.W.; Erős, T.; Finn, D.S.; Gillanders, B.M.; Hadwen, W.L.; Harrod, C.; Hermoso, V.; Jennings, S.; et al. Human effects on ecological connectivity in aquatic ecosystems: Integrating scientific approaches to support management and mitigation. Sci. Total Environ. 2015, 534, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Rudnick, D.A.; Ryan, S.J.; Beier, P.; Cushman, S.A.; Dieffenbach, F.; Epps, C.W.; Gerber, L.R.; Hartter, J.; Jenness, J.S.; Kintsch, J.; et al. The role of landscape connectivity in planning and implementing conservation and restoration priorities. Issues Ecol. 2012, 16, 1–20. [Google Scholar]
- Ferrari, J.R.; Lookingbill, T.R.; Neel, M.C. Two measures of landscape-graph connectivity: Assessment across gradients in area and configuration. Landsc. Ecol. 2007, 22, 1315–1323. [Google Scholar] [CrossRef]
- Vejrikova, I.; Vejrik, L.; Leps, J.; Kocvara, L.; Sajdlova, Z.; Ctvrtlikova, M.; Peterka, J. Impact of herbivory and competition on lake ecosystem structure: Underwater experimental manipulation. Sci. Rep. 2018, 8, 12130. [Google Scholar] [CrossRef]
- Hay, M.E.; Taylor, P.R. Competition between herbivorous fishes and urchins on Caribbean reefs. Oecologia 1985, 65, 591–598. [Google Scholar] [CrossRef]
- Humphries, A.T.; McClanahan, T.R.; McQuaid, C.D. Algal turf consumption by sea urchins and fishes is mediated by fisheries management on coral reefs in Kenya. Coral Reefs 2020, 39, 1137–1146. [Google Scholar] [CrossRef]
- Hambäck, P.A.; Beckerman, A.P. Herbivory and plant resource competition: A review of two interacting interactions. Oikos 2003, 101, 26–37. [Google Scholar] [CrossRef]
- Korzen, L.; Israel, A.; Abelson, A. Grazing effects of fish versus sea urchins on turf algae and coral recruits: Possible omplications for coral reef resilience and restoration. J. Mar. Biol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A. The impact of ecosystem connectivity on coral reef resilience. J. Appl. Ecol. 2007, 45, 854–862. [Google Scholar] [CrossRef]
- Hock, K.; Wolff, N.H.; Condie, S.A.; Anthony, K.R.N.; Mumby, P.J.; Paynter, Q. Connectivity networks reveal the risks of crown-of-thorns starfish outbreaks on the Great Barrier Reef. J. Appl. Ecol. 2014, 51, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- LeCraw, R.M.; Kratina, P.; Srivastava, D.S. Food web complexity and stability across habitat connectivity gradients. Oecologia 2014, 176, 903–915. [Google Scholar] [CrossRef]
- Wenger, S.J.; Olden, J.D. Assessing transferability of ecological models: An underappreciated aspect of statistical validation. Methods Ecol. Evol. 2012, 3, 260–267. [Google Scholar] [CrossRef]
- Beck, M.W. Inference and generality in ecology: Current problems and an experimental solution. Oikos 1997, 78, 265–273. [Google Scholar] [CrossRef]
- Loreau, M.; de Mazancourt, C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol. Lett. 2013, 16 (Suppl. 1), 106–115. [Google Scholar] [CrossRef] [PubMed]
- Côte, I.M.; Darling, E.S.; Brown, C.J. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 2016, 283, 20152592. [Google Scholar] [CrossRef] [PubMed]




| Model System | System Scale | Patch | Patch Scale | |||
|---|---|---|---|---|---|---|
| No. of Connections | Configuration | No. of Connections | Configuration | |||
| 1 |  | 1 | 18.1 | A B C D | 3 2 2 2 | 5.3 4.2 4.2 3.4 |
| 2 |  | 1 | 26.9 | A B C D | 2 2 3 2 | 4.8 3.8 6.3 4.8 |
| 3 |  | 2 | 32.7 | A B C D | 3 2 3 2 | 5.3 4.2 6.3 4.8 |
| 4 |  | 2 | 46.7 | A B C D | 3 2 3 2 | 6.7 5.0 7.1 5.3 |
| 5 |  | 2 | 48.8 | A B C D | 3 2 2 3 | 5.9 5.6 5.6 5.9 |
| 6 |  | 3 | 50.6 | A B C D | 3 2 4 2 | 6.7 5.0 8.3 5.9 |
| 7 |  | 4 | 58.3 | A B C D | 3 2 4 3 | 6.7 5.6 8.3 6.7 |
| Experiment | Pulse | Press |
|---|---|---|
| Length of experiment | 1 h | 7 h |
| No. of crabs per patch | 1 | 1 |
| No. of patches per system | 9 | 9 |
| No. of pellets added per hour to undisturbed patches (×5) | 1 | 1 |
| No. of pellets added per hour to disturbed patches (×4) | 13 | 9 |
| Experiment | Level | Connectivity Measure Tested | AIC | AICW |
|---|---|---|---|---|
| Pulse disturbance | Patch | No. of connections | 840 | 0.06 |
| Configuration | 847 | 0.06 | ||
| System | No. of connections | 967 | 0.00 | |
| Configuration | 964 | 0.00 | ||
| Additive | No. of connections(patch) + Configuration(system) | 839 | 0.10 | |
| Interaction | No. of connections(patch) × Configuration(system) | 835 | 0.77 | |
| Press disturbance | Patch | No. of connections | 1839 | 0.00 |
| Configuration | 1819 | 0.07 | ||
| System | No. of connections | 2018 | 0.00 | |
| Configuration | 2020 | 0.00 | ||
| Additive | Configuration(patch) + No. of connections(system) | 1821 | 0.03 | |
| Interaction | Configuration(patch) × No. of connections(system) | 1814 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jinks, K.I.; Brown, C.J.; Schlacher, T.A.; Olds, A.D.; Engelhard, S.L.; Pearson, R.M.; Connolly, R.M. Being Well-Connected Pays in a Disturbed World: Enhanced Herbivory in Better-Linked Habitats. Diversity 2020, 12, 424. https://doi.org/10.3390/d12110424
Jinks KI, Brown CJ, Schlacher TA, Olds AD, Engelhard SL, Pearson RM, Connolly RM. Being Well-Connected Pays in a Disturbed World: Enhanced Herbivory in Better-Linked Habitats. Diversity. 2020; 12(11):424. https://doi.org/10.3390/d12110424
Chicago/Turabian StyleJinks, Kristin I., Christopher J. Brown, Thomas A. Schlacher, Andrew D. Olds, Sarah L. Engelhard, Ryan M. Pearson, and Rod M. Connolly. 2020. "Being Well-Connected Pays in a Disturbed World: Enhanced Herbivory in Better-Linked Habitats" Diversity 12, no. 11: 424. https://doi.org/10.3390/d12110424