Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers
Abstract
:1. Introduction
2. Results
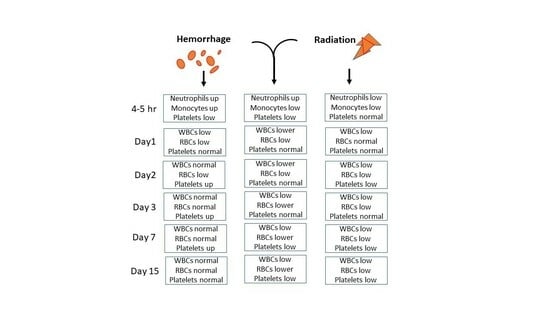
2.1. CI Reduces White Blood Cells (WBCs) More Than RI Alone on Day 1 and Day 2
2.2. CI-Induced Neutrophil Depletion Is Greater Than That of RI Alone on Day 2
2.3. CI-Induced Lymphocyte Depletion Is More Than RI Alone on Day 2
2.4. CI-Induced Monocyte Depletion Is More Than RI Alone on Day 1
2.5. CI-Induced Eosinophil Depletion Is Greater Than That of RI Alone on Day 2
2.6. CI-Induced Basophil Depletion Is Greater Than That of RI Alone on Day 2
2.7. CI Reduces Red Blood Cell Counts (RBCs) More Than RI Alone on Days 3, 7, and 15
2.8. CI Reduces Hemoglobin More Than RI Alone on Days 3, 7, and 15
2.9. CI Reduces Hematocrit More Than RI Alone on Days 3, 7, and 15
2.10. Hemo Drives Increases in Platelet Counts on Days 2, 3, and 7, but CI Does Not Reduce Platelet Counts More Than RI Alone on Days 3, 7, and 15
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Experimental Design
4.3. Gamma Irradiation
4.4. Hemorrhage (Hemo)
4.5. Assessment of Blood Cell Profile in Peripheral Blood
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Pathology of Atomic Bomb Casualties. Acta Pathol. Jpn. 1982, 32 (Suppl. 2), 237–270. [Google Scholar]
- Kishi, H.S. Effects of the “Special Bomb”: Recollections of a Neurosurgeon in Hiroshima, August 8–15, 1945. Neurosurgery 2000, 47, 441–445, discussion 45–46. [Google Scholar] [CrossRef]
- Barabanova, A.V. Significance of Beta-Radiation Skin Burns in Chernobyl Patients for the Theory and Practice of Radiopathology. Vojn. Pregl. 2006, 63, 477–480. [Google Scholar] [CrossRef]
- Kiang, J.G.; Jiao, W.; Cary, L.H.; Mog, S.R.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. Wound Trauma Increases Radiation-Induced Mortality by Activation of Inos Pathway and Elevation of Cytokine Concentrations and Bacterial Infection. Radiat. Res. 2010, 173, 319–332. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Hatchett, R.J.; Kaminski, J.M.; Ledney, G.D.; Pellmar, T.C.; Okunieff, P.; Ramakrishnan, N. Medical Countermeasures for Radiation Combined Injury: Radiation with Burn, Blast, Trauma and/or Sepsis. Report of an Niaid Workshop, March 26–27, 2007. Radiat. Res. 2008, 169, 712–721. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Swift, J.M.; Christensen, C.L.; Gupta, P.; Balakathiresan, N.; Maheshwari, R.K. Hemorrhage Exacerbates Radiation Effects on Survival, Leukocytopenia, Thrombopenia, Erythropenia, Bone Marrow Cell Depletion and Hematopoiesis, and Inflammation-Associated Micrornas Expression in Kidney. PLoS ONE 2015, 10, e0139271. [Google Scholar] [CrossRef]
- Ledney, G.D.; Elliott, T.B. Combined Injury: Factors with Potential to Impact Radiation Dose Assessments. Health Phys. 2010, 98, 145–152. [Google Scholar] [CrossRef]
- Kiang, J.G.; Garrison, B.R.; Burns, T.M.; Zhai, M.; Dews, I.C.; Ney, P.H.; Cary, L.H.; Fukumoto, R.; Elliott, T.B.; Ledney, G.D. Wound Trauma Alters Ionizing Radiation Dose Assessment. Cell Biosci. 2012, 2, 20. [Google Scholar] [CrossRef]
- Kiang, J.G.; Blakely, W.F. Combined Radiation Injury and Its Impacts on Radiation Countermeasures and Biodosimetry. Int. J. Radiat. Biol. 2023, 99, 1055–1065. [Google Scholar] [CrossRef]
- Willey, J.S.; Lloyd, S.A.; Robbins, M.E.; Bourland, J.D.; Smith-Sielicki, H.; Bowman, L.C.; Norrdin, R.W.; Bateman, T.A. Early Increase in Osteoclast Number in Mice after Whole-Body Irradiation with 2 Gy X rays. Radiat. Res. 2008, 170, 388–392. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Shi, W.G.; Li, H.; Hua, J.R.; Feng, X.; Wei, W.J.; Wang, J.F.; He, J.P.; Lei, S.W. Bone Loss Induced by Simulated Microgravity, Ionizing Radiation and/or Ultradian Rhythms in the Hindlimbs of Rats. Biomed. Environ. Sci. 2018, 31, 126–135. [Google Scholar]
- Sakurai, T.; Sawada, Y.; Yoshimoto, M.; Kawai, M.; Miyakoshi, J. Radiation-Induced Reduction of Osteoblast Differentiation in C2c12 Cells. J. Radiat. Res. 2007, 48, 515–521. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.J.; Yang, M.; Moon, C.; Kim, J.C.; Jo, S.K.; Jang, J.S.; Kim, S.H. Establishment of a Murine Model for Radiation-Induced Bone Loss Using Micro-Computed Tomography in Adult C3h/Hen Mice. Lab. Anim. Res. 2013, 29, 55–62. [Google Scholar] [CrossRef]
- Ledney, G.D.; Elliott, T.B.; Moore, M.M. Modulations of Mortality by Tissue Trauma and Sepsis in Mice after Radiation Injury. In Biological Basis of Radiation Protection Practice; Mossman, K.I., Mills, W.A., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1992; pp. 202–217. [Google Scholar]
- Valeriote, F.A.; Baker, D.G. The Combined Effects of Thermal Trauma and X-Irradiation on Early Mortality. Radiat. Res. 1964, 22, 693–702. [Google Scholar] [CrossRef]
- Manning, J.E. Fluid and Blood Resuscitation. In Emergency Medicine: A Comprehensive Study Guide; McGraw-Hill: New York, NY, USA, 2004; pp. 225–230. [Google Scholar]
- Li, Q.; Lan, X.; Han, X.; Durham, F.; Wan, J.; Weiland, A.; Koehler, R.C.; Wang, J. Microglia-Derived Interleukin-10 Accelerates Post-Intracerebral Hemorrhage Hematoma Clearance by Regulating Cd36. Brain Behav. Immun. 2021, 94, 437–457. [Google Scholar] [CrossRef]
- Rendevski, V.; Aleksovski, B.; Stojanov, D.; Aleksovski, V.; Rendevska, A.M.; Kolevska, M.; Stojanoski, K.; Gjorgoski, I. Peripheral Glutamate and Tnf-Alpha Levels in Patients with Intracerebral Hemorrhage: Their Prognostic Values and Interactions toward the Formation of the Edemal Volume. Neurol. Neurochir. Pol. 2018, 52, 207–214. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, Q.; Su, Y.; Zhang, Y.; Wang, G.; Li, F. Nf-Kappab Activation and Cell Death after Intracerebral Hemorrhage in Patients. Neurol. Sci. 2014, 35, 1097–1102. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Strong, R.; Zhang, J.; Grotta, J.C.; Aronowski, J. Distinct Patterns of Intracerebral Hemorrhage-Induced Alterations in Nf-Kappab Subunit, Inos, and Cox-2 Expression. J. Neurochem. 2007, 101, 652–663. [Google Scholar] [CrossRef]
- Takemura, N.; Kurashima, Y.; Mori, Y.; Okada, K.; Ogino, T.; Osawa, H.; Matsuno, H.; Aayam, L.; Kaneto, S.; Park, E.J.; et al. Eosinophil Depletion Suppresses Radiation-Induced Small Intestinal Fibrosis. Sci. Transl. Med. 2018, 10, eaan0333. [Google Scholar] [CrossRef]
- Cromheecke, J.L.; Nguyen, K.T.; Huston, D.P. Emerging Role of Human Basophil Biology in Health and Disease. Curr. Allergy Asthma Rep. 2014, 14, 408. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kato, T.; Naito, T.; Tanei, T.; Okada, K.; Ito, R.; Koketsu, Y.; Hirayama, K. Long-Term Risks of Hemorrhage and Adverse Radiation Effects of Stereotactic Radiosurgery for Brain Arteriovenous Malformations. Neurosurgery 2022, 90, 784–792. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological Effects of Ionizing Radiation: Endothelial Activation and Dysfunction. Cell Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of Radiation-Induced Endothelium Damage: Emerging Models and Technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Elliott, T.B.; Gupta, P.; Balakathiresan, N.S.; Maheshwari, R.K.; Knollmann-Ritschel, B. Hemorrhage Enhances Cytokine, Complement Component 3, and Caspase-3, and Regulates Micrornas Associated with Intestinal Damage after Whole-Body Gamma-Irradiation in Combined Injury. PLoS ONE 2017, 12, e0184393. [Google Scholar] [CrossRef]
- Maugeri, N.; Evangelista, V.; Celardo, A.; Dell’Elba, G.; Martelli, N.; Piccardoni, P.; de Gaetano, G.; Cerletti, C. Polymorphonuclear Leukocyte-Platelet Interaction: Role of P-Selectin in Thromboxane B2 and Leukotriene C4 Cooperative Synthesis. Thromb. Haemost. 1994, 72, 450–456. [Google Scholar] [CrossRef]
- Maugeri, N.; Rovere-Querini, P.; Evangelista, V.; Covino, C.; Capobianco, A.; Bertilaccio, M.T.; Piccoli, A.; Totani, L.; Cianflone, D.; Maseri, A.; et al. Neutrophils Phagocytose Activated Platelets In Vivo: A Phosphatidylserine, P-Selectin, and Beta2 Integrin-Dependent Cell Clearance Program. Blood 2009, 113, 5254–5265. [Google Scholar] [CrossRef]
- Page, C.; Pitchford, S. Neutrophil and Platelet Complexes and Their Relevance to Neutrophil Recruitment and Activation. Int. Immunopharmacol. 2013, 17, 1176–1184. [Google Scholar] [CrossRef]
- Cunin, P.; Bouslama, R.; Machlus, K.R.; Martinez-Bonet, M.; Lee, P.Y.; Wactor, A.; Nelson-Maney, N.; Morris, A.; Guo, L.; Weyrich, A.; et al. Megakaryocyte Emperipolesis Mediates Membrane Transfer from Intracytoplasmic Neutrophils to Platelets. eLife 2019, 8, e44031. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Manfredi, A.A.; Maugeri, N. Misunderstandings between Platelets and Neutrophils Build in Chronic Inflammation. Front. Immunol. 2019, 10, 2491. [Google Scholar] [CrossRef]
- Zawaski, J.A.; Yates, C.R.; Miller, D.D.; Kaffes, C.C.; Sabek, O.M.; Afshar, S.F.; Young, D.A.; Yang, Y.; Gaber, M.W. Radiation Combined Injury Models to Study the Effects of Interventions and Wound Biomechanics. Radiat. Res. 2014, 182, 640–652. [Google Scholar] [CrossRef]
- Chino, Y.; Olson, J.D.; Schaaf, G.W.; Cline, J.M.; Johnson, T.E. Longitudinal Analysis of Leukocyte Total and Differential Count of Rhesus Macaques (Macaca Mulatta) after Total-Body Irradiation. Radiat. Res. 2023, 200, 349–356. [Google Scholar] [CrossRef]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. Delta-Tocotrienol Protects Mouse and Human Hematopoietic Progenitors from Gamma-Irradiation through Extracellular Signal-Regulated Kinase/Mammalian Target of Rapamycin Signaling. Haematologica 2010, 95, 1996–2004. [Google Scholar] [CrossRef]
- Golde, W.T.; Gollobin, P.; Rodriguez, L.L. A Rapid, Simple, and Humane Method for Submandibular Bleeding of Mice Using a Lancet. Lab Anim. 2005, 34, 39–43. [Google Scholar] [CrossRef]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C.; Association European Federation of Pharmaceutical Industries, and Methods European Centre for the Validation of Alternative. A Good Practice Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef]







| Cell Type | Condition | 4–5 h | 1D | 2D | 3D | 7D | 15D |
|---|---|---|---|---|---|---|---|
| WBCs | Hemo | − | ↓ | − | − | − | − |
| RI | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓↓ | ↓↓ | ↓ | ↓ | ↓ | |
| NEU | Hemo | ↑ | − | − | − | − | − |
| RI | ↓ | − | ↓ | ↓ | ↓ | ↓ | |
| CI | ↑ | − | ↓↓ | ↓ | ↓ | ↓ | |
| LYM | Hemo | ↓ | ↓ | − | − | − | − |
| RI | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓ | ↓↓ | ↓ | ↓ | ↓ | |
| MONO | Hemo | ↑ | ↓ | − | − | − | − |
| RI | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓↓ | ↓ | ↓ | ↓ | ↓ | |
| EOS | Hemo | − | − | − | − | ↓ | − |
| RI | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓ | ↓↓ | ↓ | ↓ | ↓ | |
| BASO | Hemo | ↓ | − | − | − | − | − |
| RI | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓ | ↓↓ | ↓ | ↓ | ↓ | |
| RBCs | Hemo | ↓ | ↓ | ↓ | − | − | − |
| RI | − | − | − | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓ | ↓ | ↓↓ | ↓↓ | ↓↓ | |
| HGB | Hemo | ↓ | ↓ | ↓ | ↓ | − | − |
| RI | − | − | ↓ | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| HCT | Hemo | ↓ | ↓ | ↓ | ↓ | − | − |
| RI | − | − | − | ↓ | ↓ | ↓ | |
| CI | ↓ | ↓ | ↓ | ↓↓ | ↓↓ | ↓↓ | |
| PLT | Hemo | ↓ | − | ↑ | ↑ | ↑ | − |
| RI | − | − | ↓ | − | ↓ | ↓ | |
| CI | ↓ | − | − | − | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiang, J.G.; Woods, A.K.; Cannon, G. Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers. Int. J. Mol. Sci. 2024, 25, 2988. https://doi.org/10.3390/ijms25052988
Kiang JG, Woods AK, Cannon G. Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers. International Journal of Molecular Sciences. 2024; 25(5):2988. https://doi.org/10.3390/ijms25052988
Chicago/Turabian StyleKiang, Juliann G., Akeylah K. Woods, and Georgetta Cannon. 2024. "Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers" International Journal of Molecular Sciences 25, no. 5: 2988. https://doi.org/10.3390/ijms25052988