Resistance and Aerobic Training Were Effective in Activating Different Markers of the Browning Process in Obesity
Abstract
:1. Introduction
2. Results
2.1. Effect of the High-Calorie, High-Fat Diet on the Induction of Obesity
2.2. Progression of the Load and Performance in the Training Groups
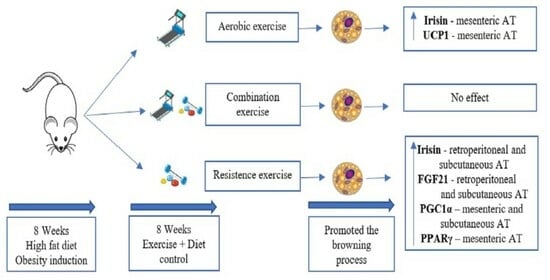
2.3. Adipose Tissue Browning Mediated by Physical Training
3. Discussion
4. Material and Methods
4.1. Animals, Diet, and Experiment Groups
4.2. Body Mass Gain and Feed Consumption
4.3. Training Protocol
4.3.1. Test Description and Strength Training
4.3.2. Effort Test and Aerobic Training
4.3.3. Combination Training
4.4. Euthanasia
4.5. Assessment of ELISA
4.6. Protein Extraction and Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Aerobic exercise group |
| AT | Adipose tissue |
| BAT | Brown adipose tissue |
| CE | Combination exercise group |
| CS | Sedentary control diet group |
| FGF21 | Fibroblast growth factor 21 |
| HS | Sedentary high-fat diet group |
| PGC1α | Coactivator 1α |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RE | Resistance exercise group |
| UCP1 | Uncoupling protein 1 |
| WAT | White adipose tissue |
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Brazil Ministry of Health. Health Surveillance Department; Department of Health Analysis and Surveillance of Non-Communicable Diseases, Ministry of Health: Brasilia, Brazil, 2021; 126p. [Google Scholar]
- Corgosinho, F.C.; Ackel-D’Elia, C.; Tufik, S.; Dâmaso, A.R.; de Piano, A.; Sanches Pde, L.; Campos, R.M.; Silva, P.L.; Carnier, J.; Tock, L.; et al. Beneficial effects of a multifaceted 1-year lifestyle intervention on metabolic abnormalities in obese adolescents with and without sleep-disordered breathing. Metab. Syndr. Relat. Disord. 2015, 13, 110–118. [Google Scholar] [CrossRef]
- Hsu, K.J.; Liao, C.D.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, T.; Goel, K.; Corrêa de Sá, D.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; Park, J.S.; Kober, L.; Torp-Pedersen, C.; Cottin, Y.; et al. Central obesity and survival in subjects with coronary artery disease: A systematic review of the literature and collaborative analysis with individual subject data. J. Am. Coll. Cardiol. 2011, 57, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids. 2005, 73, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. New powers of brown fat: Fighting the metabolic syndrome. Cell Metab. 2011, 13, 238–240. [Google Scholar] [CrossRef]
- Sartini, L.; Frontini, A. Potential novel therapeutic strategies from understanding adipocyte transdifferentiation mechanisms. Expert. Rev. Endocrinol. Metab. 2015, 10, 143–152. [Google Scholar] [CrossRef]
- Di Maio, G.; Alessio, N.; Demirsoy, I.H.; Peluso, G.; Perrotta, S.; Monda, M.; Di Bernardo, G. Evaluation of Browning Agents on the White Adipogenesis of Bone Marrow Mesenchymal Stromal Cells: A Contribution to Fighting Obesity. Cells 2021, 10, 403. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L.; Williams, T.D.; Dobbs, W.C. The Effect of Chronic Exercise Training on Leptin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2018, 48, 1437–1450. [Google Scholar] [CrossRef]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23 (Suppl. S1), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Pink Adipocytes. Trends Endocrinol. Metab. 2018, 29, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, A.; Stanford, K. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Ahmadabadi, F.; Nakhaei, H.; Mogharnasi, M.; Huang, C.J. Aerobic interval training improves irisin and chemerin levels of both liver and visceral adipose tissues and circulating asprosin in rats with metabolic syndrome. Physiol. Int. 2021, 108, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Rodrigues, S.; Rodríguez, A.; Gouveia, A.M.; Gonçalves, I.O.; Becerril, S.; Ramírez, B.; Beleza, J.; Frühbeck, G.; Ascensão, A.; Magalhães, J. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci. 2016, 165, 100–108. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; So, B.; Son, J.S.; Yoon, D.; Song, W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: A pilot study. Physiol. Res. 2016, 65, 271–279. [Google Scholar] [CrossRef]
- Abdi, A.; Mehrabani, J.; Nordvall, M.; Wong, A.; Fallah, A.; Bagheri, R. Effects of concurrent training on irisin and fibronectin type-III domain containing 5 (FNDC5) expression in visceral adipose tissue in type-2 diabetic rats. Arch. Physiol. Biochem. 2022, 128, 651–656. [Google Scholar] [CrossRef]
- Atakan, M.M.; Koşar, Ş.N.; Güzel, Y.; Tin, H.T.; Yan, X. The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liao, B.; Jin, L.; Huang, Z.; Triggle, C.R.; Ding, H.; Zhang, J.; Huang, Y.; Lin, Z.; Xu, A. Exercise Alleviates Obesity-Induced Metabolic Dysfunction via Enhancing FGF21 Sensitivity in Adipose Tissues. Cell Rep. 2019, 26, 2738–2752.e4. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Sahl, R.E.; Trammell, S.A.; Svenningsen, J.S.; Gillum, M.P.; Helge, J.W.; Clemmensen, C. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight. 2018, 3, e122737. [Google Scholar] [CrossRef]
- Motahari, R.M.; Bijeh, N.; Attarzadeh, H.S.; Raouf, S.A. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morton, T.L.; Galior, K.; McGrath, C.; Wu, X.; Uzer, G.; Uzer, G.B.; Sen, B.; Xie, Z.; Tyson, D.; Rubin, J.; et al. Exercise Increases and Browns Muscle Lipid in High-Fat Diet-Fed Mice. Front. Endocrinol. 2016, 7, 80. [Google Scholar] [CrossRef]
- Aldiss, P.; Lewis, J.E.; Lupini, I.; Bloor, I.; Chavoshinejad, R.; Boocock, D.J.; Miles, A.K.; Ebling, F.J.P.; Budge, H.; Symonds, M.E. Exercise Training in Obese Rats Does Not Induce Browning at Thermoneutrality and Induces a Muscle-Like Signature in Brown Adipose Tissue. Front. Endocrinol. 2020, 11, 97. [Google Scholar] [CrossRef]
- Vosselman, M.J.; Hoeks, J.; Brans, B.; Pallubinsky, H.; Nascimento, E.B.; van der Lans, A.A.; Broeders, E.P.; Mottaghy, F.M.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. 2015, 39, 1696–1702. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Jianfei, C.; Xiaodong, X.; Yuanyuan, D.; Jing, Z. Effects of different exercise intensity on PPARγ and relative index in adolescent obesity rats. Wei Sheng Yan Jiu 2014, 43, 732–737. [Google Scholar] [PubMed]
- Picoli, C.C.; Gilio, G.R.; Henriques, F.; Leal, L.G.; Besson, J.C.; Lopes, M.A.; Franzói de Moraes, S.M.; Hernandes, L.; Batista Junior, M.L.; Peres, S.B. Resistance exercise training induces subcutaneous and visceral adipose tissue browning in Swiss mice. J. Appl. Physiol. 2020, 129, 66–74. [Google Scholar] [CrossRef]
- Ziegler, A.K.; Damgaard, A.; Mackey, A.L.; Schjerling, P.; Magnusson, P.; Olesen, A.T.; Kjaer, M.; Scheele, C. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci. Rep. 2019, 9, 12069. [Google Scholar] [CrossRef] [PubMed]
- Stotzer, U.S.; Rodrigues, M.F.; Domingos, M.M.; Silva, G.H.; Duarte, F.O.; Gatto, C.V.; Duarte, A.C.; Shiguemoto, G.E.; Perez, S.E.; Selistre-de-Araujo, H.S. Resistance training suppresses intra-abdominal fatty acid synthesis in ovariectomized rats. Int. J. Sports Med. 2015, 36, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Stinkens, R.; Brouwers, B.; Jocken, J.W.; Blaak, E.E.; Teunissen-Beekman, K.F.; Hesselink, M.K.; van Baak, M.A.; Schrauwen, P.; Goossens, G.H. Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J. Appl. Physiol. 2018, 125, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.J.; Zhu, J.Y.; Chen, M.; Guo, L. Exercise-Mediated Browning of White Adipose Tissue: Its Significance, Mechanism and Effectiveness. Int. J. Mol. Sci. 2021, 22, 11512. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Qin, M.; Wang, P.; Li, S.; Wang, X. Regulatory effects and mechanisms of exercise on activation of brown adipose tissue (BAT) and browning of white adipose tissue (WAT). Adipocyte 2023, 12, 2266147. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Giordano, A.; Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 2009, 214, 171–178. [Google Scholar] [CrossRef]
- Efremova, A.; Senzacqua, M.; Venema, W.; Isakov, E.; Di Vincenzo, A.; Zingaretti, M.C.; Protasoni, M.; Thomski, M.; Giordano, A.; Cinti, S. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J. Physiol. Biochem. 2020, 76, 185–192. [Google Scholar] [CrossRef]
- Jimenez, M.; Barbatelli, G.; Allevi, R.; Cinti, S.; Seydoux, J.; Giacobino, J.P.; Muzzin, P.; Preitner, F. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 2003, 270, 699–705. [Google Scholar] [CrossRef]
- Verheggen, R.; Maessen, M.; Green, D.; Hermus, A.; Hopman, M.; Thijssen, D. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: Distinct effects on body weight and visceral adipose tissue. Obes. Ver. 2016, 17, 664–690. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, T.; Zhou, P.; Li, R.; Liu, Z.; Xie, J.; Hua, T.; Sun, Q. Exercise ameliorates insulin resistance and improves ASK1-mediated insulin signalling in obese rats. J. Cell Mol. Med. 2021, 25, 10930–10938. [Google Scholar] [CrossRef] [PubMed]
- Boldarine, V.T.; Pedroso, A.P.; Neto, N.I.P.; Dornellas, A.P.S.; Nascimento, C.M.O.; Oyama, L.M.; Ribeiro, E.B. High-fat diet intake induces depressive-like behavior in ovariectomized rats. Sci. Rep. 2019, 9, 10551. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, I.S.; Zemdegs, J.C.; de Souza, A.P.; Watanabe, R.L.; Telles, M.M.; Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B. Diet-induced obesity impairs hypothalamic glucose sensing but not glucose hypothalamic extracellular levels, as measured by microdialysis. Nutr. Diabetes 2015, 5, e162. [Google Scholar] [CrossRef] [PubMed]
- Mônico-Neto, M.; Antunes, H.K.; Lee, K.S.; Phillips, S.M.; Giampá, S.Q.; Souza Hde, S.; Dáttilo, M.; Medeiros, A.; de Moraes, W.M.; Tufik, S.; et al. Resistance training minimizes catabolic effects induced by sleep deprivation in rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1143–1150. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontes, L.P.P.; Alves Nakakura, F.C.; Neto, N.I.P.; Boldarine, V.T.; Maza, P.K.; Santos, P.F.; Avila, F.; Silva-Neto, A.F.; Antunes, H.K.M.; Dâmaso, A.R.; et al. Resistance and Aerobic Training Were Effective in Activating Different Markers of the Browning Process in Obesity. Int. J. Mol. Sci. 2024, 25, 275. https://doi.org/10.3390/ijms25010275
Pontes LPP, Alves Nakakura FC, Neto NIP, Boldarine VT, Maza PK, Santos PF, Avila F, Silva-Neto AF, Antunes HKM, Dâmaso AR, et al. Resistance and Aerobic Training Were Effective in Activating Different Markers of the Browning Process in Obesity. International Journal of Molecular Sciences. 2024; 25(1):275. https://doi.org/10.3390/ijms25010275
Chicago/Turabian StylePontes, Lidia Passinho Paz, Fernanda Cristina Alves Nakakura, Nelson Inácio Pinto Neto, Valter Tadeu Boldarine, Paloma Korehisa Maza, Paloma Freire Santos, Felipe Avila, Artur Francisco Silva-Neto, Hanna Karen Moreira Antunes, Ana Raimunda Dâmaso, and et al. 2024. "Resistance and Aerobic Training Were Effective in Activating Different Markers of the Browning Process in Obesity" International Journal of Molecular Sciences 25, no. 1: 275. https://doi.org/10.3390/ijms25010275