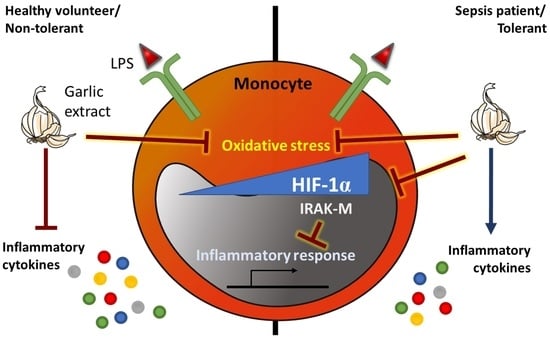
Thiosulfinate-Enriched Allium sativum Extract Exhibits Differential Effects between Healthy and Sepsis Patients: The Implication of HIF-1α
Abstract
:1. Introduction
2. Results
2.1. Thiosulfinate-Enriched Allium sativum Extract Reduces Lipopolysaccharide-Triggered Oxidative Stress in Monocytes from Both Patients with Sepsis and Healthy Volunteers
2.2. Thiosulfinate-Enriched Allium sativum Extract Exhibits Divergent Effects on Monocytes from Patients with Sepsis Compared with Healthy Volunteers
2.3. Thiosulfinate-Enriched Allium sativum Extract Increases the Lipopolysaccharide Response of Monocytes with an Endotoxin-Tolerant Phenotype
2.4. Thiosulfinate-Enriched Allium sativum Extract Reduces HIF-1α Pathway Activation in Sepsis Monocytes
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Thiosulfinate-Enriched Allium sativum Extract Preparation
4.3. Human Monocyte Isolation
4.4. In Vitro Stimulation
4.5. Cell Reactive Oxygen Species Measurement
4.6. Flow Cytometry Characterization
4.7. Inflammatory Cytokine Quantification
4.8. Dimethyloxalylglycine In Vitro Model
4.9. Relative mRNA Expression Quantification
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Saber Batiha, G.; Magdy Beshbishy, A.; G Wasef, L.; Elewa, Y.H.A.; A Al-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; M Abd-Elhakim, Y.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Ortiz, J.M.; Galan-Moya, E.M.; de la Cruz-Morcillo, M.A.; Rodriguez, J.F.; Gracia, I.; Garcia, M.T.; Redondo-Calvo, F.J. Cost Effective Use of a Thiosulfinate-Enriched Allium Sativum Extract in Combination with Chemotherapy in Colon Cancer. Int. J. Mol. Sci. 2020, 21, 2766. [Google Scholar] [CrossRef]
- Wei, Z.; Shan, Y.; Tao, L.; Liu, Y.; Zhu, Z.; Liu, Z.; Wu, Y.; Chen, W.; Wang, A.; Lu, Y. Diallyl Trisulfides, a Natural Histone Deacetylase Inhibitor, Attenuate HIF-1α Synthesis, and Decreases Breast Cancer Metastasis. Mol. Carcinog. 2017, 56, 2317–2331. [Google Scholar] [CrossRef]
- Pandey, N.; Tyagi, G.; Kaur, P.; Pradhan, S.; Rajam, M.V.; Srivastava, T. Allicin Overcomes Hypoxia Mediated Cisplatin Resistance in Lung Cancer Cells through ROS Mediated Cell Death Pathway and by Suppressing Hypoxia Inducible Factors. Cell. Physiol. Biochem. 2020, 54, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Arellano-Buendía, A.S.; Castañeda-Lara, L.G.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Rojas-Morales, P.; Argüello-García, R.; Juárez-Rojas, J.G.; Tapia, E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants 2020, 9, 1134. [Google Scholar] [CrossRef]
- Lin, K.-H.; Wei, Y.-M.; Liu, C.-H.; Liu, J.-S.; Huang, I.-C.; Viswanadha, V.P.; Huang, C.-Y.; Kuo, W.-W. Diallyl Trisulfide Suppresses High-Glucose-Induced Cardiomyocyte Apoptosis by Targeting Reactive Oxygen Species-Mediated Hypoxia-Inducible Factor-1α/Insulin-like Growth Factor Binding Protein 3 Activation. J. Agric. Food Chem. 2021, 69, 11696–11708. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Ortuño-Sahagún, D.; Vázquez-Carrera, M.; López-Roa, R.I. Alliin, a Garlic (Allium sativum) Compound, Prevents LPS-Induced Inflammation in 3T3-L1 Adipocytes. Mediat. Inflamm. 2013, 2013, e381815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, A.; Thornton, C.E.; Xu, B.; Hennessy, A. Garlic Increases IL-10 and Inhibits TNFalpha and IL-6 Production in Endotoxin-Stimulated Human Placental Explants. Placenta 2005, 26, 828–834. [Google Scholar] [CrossRef]
- Keiss, H.-P.; Dirsch, V.M.; Hartung, T.; Haffner, T.; Trueman, L.; Auger, J.; Kahane, R.; Vollmar, A.M. Garlic (Allium sativum L.) Modulates Cytokine Expression in Lipopolysaccharide-Activated Human Blood Thereby Inhibiting NF-KappaB Activity. J. Nutr. 2003, 133, 2171–2175. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.; Lahav, M.; Sakhnini, E.; Barshack, I.; Fidder, H.H.; Avidan, B.; Bardan, E.; Hershkoviz, R.; Bar-Meir, S.; Chowers, Y. Allicin Inhibits Spontaneous and TNF-Alpha Induced Secretion of Proinflammatory Cytokines and Chemokines from Intestinal Epithelial Cells. Clin. Nutr. 2004, 23, 1199–1208. [Google Scholar] [CrossRef]
- Deng, X.; Yang, P.; Gao, T.; Liu, M.; Li, X. Allicin Attenuates Myocardial Apoptosis, Inflammation and Mitochondrial Injury during Hypoxia-Reoxygenation: An in Vitro Study. BMC Cardiovasc. Disord. 2021, 21, 200. [Google Scholar] [CrossRef]
- Xu, C.; Mathews, A.E.; Rodrigues, C.; Eudy, B.J.; Rowe, C.A.; O’Donoughue, A.; Percival, S.S. Aged Garlic Extract Supplementation Modifies Inflammation and Immunity of Adults with Obesity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin. Nutr. ESPEN 2018, 24, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin Enhances Host Pro-Inflammatory Immune Responses and Protects against Acute Murine Malaria Infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.S.; Moon, E.Y.; Cho, C.G.; Pyo, S. Immunomodulating Effect of Garlic Component, Allicin, on Murine Peritoneal Macrophages. Nutr. Res. 2001, 21, 617–626. [Google Scholar] [CrossRef]
- Islam, N.; Iqbal, J.; Hasan, N.; Fatima, Z.; Thakur, H.; Hasan, N.; Mahdi, A.A.; Khan, T.; Bano, F.; Ansari, I.A. Allicin from Garlic Suppresses TNF-α and Augments IFN-γ Expressions in Monocyte Cultures from Patients with Vaginitis. Nat. Prec. 2008. [Google Scholar] [CrossRef]
- Redondo-Calvo, F.J.; Bejarano-Ramírez, N.; Baladrón, V.; Montenegro, O.; Gómez, L.A.; Velasco, R.; Villasanti, N.; Illescas, S.; Franco-Sereno, M.T.; Gracia, I.; et al. Black Garlic and Thiosulfinate-Enriched Extracts as Adjuvants to Ceftriaxone Treatment in a Rat Peritonitis Model of Sepsis. Biomedicines 2022, 10, 3095. [Google Scholar] [CrossRef]
- Schroder, K.; Irvine, K.M.; Taylor, M.S.; Bokil, N.J.; Le Cao, K.-A.; Masterman, K.-A.; Labzin, L.I.; Semple, C.A.; Kapetanovic, R.; Fairbairn, L.; et al. Conservation and Divergence in Toll-like Receptor 4-Regulated Gene Expression in Primary Human versus Mouse Macrophages. Proc. Natl. Acad. Sci. USA 2012, 109, E944–E953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Coopersmith, C.M.; McDunn, J.E.; Ferguson, T.A. The Sepsis Seesaw: Tilting toward Immunosuppression. Nat. Med. 2009, 15, 496–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Yu, M.; Chai, Y. Pathological Alteration and Therapeutic Implications of Sepsis-Induced Immune Cell Apoptosis. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cavaillon, J.-M.; Adib-Conquy, M. Bench-to-Bedside Review: Endotoxin Tolerance as a Model of Leukocyte Reprogramming in Sepsis. Crit. Care 2006, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avendaño-Ortiz, J.; Maroun-Eid, C.; Martín-Quirós, A.; Lozano-Rodríguez, R.; Llanos-González, E.; Toledano, V.; Gómez-Campelo, P.; Montalbán-Hernández, K.; Carballo-Cardona, C.; Aguirre, L.A.; et al. Oxygen Saturation on Admission Is a Predictive Biomarker for PD-L1 Expression on Circulating Monocytes and Impaired Immune Response in Patients With Sepsis. Front. Immunol. 2018, 9, 2008. [Google Scholar] [CrossRef]
- Avendaño-Ortiz, J.; Maroun-Eid, C.; Martín-Quirós, A.; Toledano, V.; Cubillos-Zapata, C.; Gómez-Campelo, P.; Varela-Serrano, A.; Casas-Martin, J.; Llanos-González, E.; Alvarez, E.; et al. PD-L1 Overexpression During Endotoxin Tolerance Impairs the Adaptive Immune Response in Septic Patients via HIF1α. J. Infect. Dis. 2018, 217, 393–404. [Google Scholar] [CrossRef]
- Shalova, I.N.; Lim, J.Y.; Chittezhath, M.; Zinkernagel, A.S.; Beasley, F.; Hernández-Jiménez, E.; Toledano, V.; Cubillos-Zapata, C.; Rapisarda, A.; Chen, J.; et al. Human Monocytes Undergo Functional Re-Programming during Sepsis Mediated by Hypoxia-Inducible Factor-1α. Immunity 2015, 42, 484–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Maldifassi, M.C.; Atienza, G.; Arnalich, F.; López-Collazo, E.; Cedillo, J.L.; Martín-Sánchez, C.; Bordas, A.; Renart, J.; Montiel, C. A New IRAK-M-Mediated Mechanism Implicated in the Anti-Inflammatory Effect of Nicotine via A7 Nicotinic Receptors in Human Macrophages. PLoS ONE 2014, 9, e108397. [Google Scholar] [CrossRef] [Green Version]
- del Fresno, C.; Soler-Rangel, L.; Soares-Schanoski, A.; Gómez-Piña, V.; González-León, M.C.; Gómez-García, L.; Mendoza-Barberá, E.; Rodríguez-Rojas, A.; García, F.; Fuentes-Prior, P.; et al. Inflammatory Responses Associated with Acute Coronary Syndrome Up-Regulate IRAK-M and Induce Endotoxin Tolerance in Circulating Monocytes. J. Endotoxin Res. 2007, 13, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Shu, Y.; Cui, T.; Fu, P. Allicin Inhibits Human Renal Clear Cell Carcinoma Progression via Suppressing HIF Pathway. Int. J. Clin. Exp. Med. 2015, 8, 20573–20580. [Google Scholar]
- Mousa, A.M.; Soliman, K.E.; Alhumaydhi, F.; Almatroudi, A.; Rugaie, O.A.; Allemailem, K.S.; Alrumaihi, F.; Khan, A.; Rezk, M.Y.; Aljasir, M.; et al. Garlic Extract Alleviates Trastuzumab-Induced Hepatotoxicity in Rats Through Its Antioxidant, Anti-Inflammatory, and Antihyperlipidemic Effects. JIR 2021, 14, 6305–6316. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, e401630. [Google Scholar] [CrossRef] [Green Version]
- Bayraktar, O.; Tekin, N.; Aydın, O.; Akyuz, F.; Musmul, A.; Burukoglu, D. Effects of S-Allyl Cysteine on Lung and Liver Tissue in a Rat Model of Lipopolysaccharide-Induced Sepsis. Naunyn-Schmiedebergs Arch. Pharmacol. 2015, 388, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Park, Y.J.; Ko, M.J.; Wang, Z.; Lee, H.Y.; Choi, Y.W.; Bae, Y.-S. A Novel Natural Compound from Garlic (Allium sativum L.) with Therapeutic Effects against Experimental Polymicrobial Sepsis. Biochem. Biophys. Res. Commun. 2015, 464, 774–779. [Google Scholar] [CrossRef]
- Redondo-Calvo, F.J.; Montenegro, O.; Padilla-Valverde, D.; Villarejo, P.; Baladrón, V.; Bejarano-Ramírez, N.; Galán, R.; Gómez, L.A.; Villasanti, N.; Illescas, S.; et al. Thiosulfinate-Enriched Allium Sativum Extract as an Adjunct to Antibiotic Treatment of Sepsis in a Rat Peritonitis Model. Appl. Sci. 2021, 11, 4760. [Google Scholar] [CrossRef]
- Santos, S.S.; Carmo, A.M.; Brunialti, M.K.C.; Machado, F.R.; Azevedo, L.C.; Assunção, M.; Trevelin, S.C.; Cunha, F.Q.; Salomao, R. Modulation of Monocytes in Septic Patients: Preserved Phagocytic Activity, Increased ROS and NO Generation, and Decreased Production of Inflammatory Cytokines. Intensive Care Med. Exp. 2016, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Lu, J.; Yang, S.; Chen, Y.; Yu, J.; Guan, S. Alliin Alleviates LPS-Induced Pyroptosis via Promoting Mitophagy in THP-1 Macrophages and Mice. Food Chem. Toxicol. 2022, 160, 112811. [Google Scholar] [CrossRef] [PubMed]
- Escoll, P.; del Fresno, C.; García, L.; Vallés, G.; Lendínez, M.J.; Arnalich, F.; López-Collazo, E. Rapid Up-Regulation of IRAK-M Expression Following a Second Endotoxin Challenge in Human Monocytes and in Monocytes Isolated from Septic Patients. Biochem. Biophys. Res. Commun. 2003, 311, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A.; Medzhitov, R.; Flavell, R.A. IRAK-M Is a Negative Regulator of Toll-like Receptor Signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef] [Green Version]
- van ’t Veer, C.; van den Pangaart, P.S.; van Zoelen, M.A.D.; de Kruif, M.; Birjmohun, R.S.; Stroes, E.S.; de Vos, A.F.; van der Poll, T. Induction of IRAK-M Is Associated with Lipopolysaccharide Tolerance in a Human Endotoxemia Model. J. Immunol. 2007, 179, 7110–7120. [Google Scholar] [CrossRef] [Green Version]
- Aminova, L.R.; Siddiq, A.; Ratan, R.R. Antioxidants, HIF Prolyl Hydroxylase Inhibitors or Short Interfering RNAs to BNIP3 or PUMA, Can Prevent Prodeath Effects of the Transcriptional Activator, HIF-1α, in a Mouse Hippocampal Neuronal Line. Antioxid. Redox Signal. 2008, 10, 1989–1998. [Google Scholar] [CrossRef] [Green Version]
- Yun, B.D.; Son, S.W.; Choi, S.Y.; Kuh, H.J.; Oh, T.-J.; Park, J.K. Anti-Cancer Activity of Phytochemicals Targeting Hypoxia-Inducible Factor-1 Alpha. Int. J. Mol. Sci. 2021, 22, 9819. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Hernández-Jiménez, E.; Toledano, V.; Esteban-Burgos, L.; Fernández-Ruíz, I.; Gómez-Piña, V.; Del Fresno, C.; Siliceo, M.; Prieto-Chinchiña, P.; Pérez de Diego, R.; et al. NFκB2/P100 Is a Key Factor for Endotoxin Tolerance in Human Monocytes: A Demonstration Using Primary Human Monocytes from Patients with Sepsis. J. Immunol. 2014, 193, 4195–4202. [Google Scholar] [CrossRef] [Green Version]
- del Fresno, C.; García-Rio, F.; Gómez-Piña, V.; Soares-Schanoski, A.; Fernández-Ruíz, I.; Jurado, T.; Kajiji, T.; Shu, C.; Marín, E.; Gutierrez del Arroyo, A.; et al. Potent Phagocytic Activity with Impaired Antigen Presentation Identifying Lipopolysaccharide-Tolerant Human Monocytes: Demonstration in Isolated Monocytes from Cystic Fibrosis Patients. J. Immunol. 2009, 182, 6494–6507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Chiang, T.-H.; Sun, Y.-Y.; Lin, M.-S. Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model. Nutrients 2019, 11, 700. [Google Scholar] [CrossRef] [Green Version]





| Controls (n = 15) | Sepsis (n = 7) | p-Value | |
|---|---|---|---|
| Age—years | 51.53 ± 5.21 | 56 ± 22.8 | 0.57 |
| Sex, male—n (%) | 9 (60) | 4 (57.1) | 0.99 |
| Comorbidities | |||
| Hypertension | 1 (6.7) | 2 (28.6) | 0.23 |
| Diabetes Mellitus | 1 (6.7) | 1 (14.3) | 0.99 |
| COPD | 1 (6.7) | 2 (28.6) | 0.23 |
| Current smoker | 2 (15.4) | 2 (28.6) | 0.56 |
| Temperature—°C | 36.5 ± 0.74 | 37.66 ± 1.6 | 0.03 * |
| HR—bpm | 84.64 ± 22.7 | 92.29 ± 18.6 | 0.44 |
| RR—bpm | 15.1 ± 2.5 | 19.14 ± 4.6 | 0.03 * |
| SBP—mm Hg | 124.7 ± 33.1 | 120 ± 43.87 | 0.78 |
| MBP—mm Hg | 86.4 ± 12.3 | 81.52 ± 24.6 | 0.54 |
| Leukocytes—103/µL | 6.94 ± 1.2 | 12.2 ± 5.83 | 0.01 * |
| Neutrophils—103/µL | 4.18 ± 1.33 | 10.25 ± 5.69 | 0.01 * |
| Lymphocytes—103/µL | 2.27 ± 0.66 | 1.18 ± 0.41 | <0.01 ** |
| Monocytes—103/µL | 0.48 ± 0.13 | 0.42 ± 0.23 | 0.25 |
| Platelets—103/µL | 236.8 ± 45.1 | 205.9 ± 88.3 | 0.72 |
| Creatinine—mg/dL | 0.91 ± 0.17 | 1.54 ± 1.37 | 0.08 |
| CRP—mg/L | 1.35 ± 0.9 | 136.4 ± 93.5 | <0.001 *** |
| Bilirubin—mg/dL | 0.73 ± 0.41 | 0.42 ± 0.18 | 0.1 |
| qSOFA | - | 0.81 ± 0.73 | - |
| Compound | TASE (mg/g) |
|---|---|
| Total Polyphenols | 13.91 |
| Total Flavonoids | 3.22 |
| Diallyl thiosulfinate (allicin) | 7.03 |
| S-allyl-L-cysteine | 0.08 |
| Leucine | 0.586 |
| Isoleucine | 0.500 |
| Valine | 0.477 |
| Methionine | 0.316 |
| Cysteine | 0.811 |
| Phenylalanine | 0.556 |
| Tyrosine | 4.499 |
| Aspartic Acid | 0.901 |
| Glutamic Acid | 2.866 |
| Arginine | 4.090 |
| Lysine | 0.617 |
| Histidine | 0.891 |
| Threonine | 0.812 |
| Serine | 0.385 |
| Glycine | 0.215 |
| Alanine | 0.897 |
| Thiamine (B1) | 0.552 |
| Riboflavin (B2) | 0.002 |
| Niacin (B3) | 0.026 |
| Pantothenic acid (B5) | 1.556 |
| Biotin (B7) | 0.251 |
| Cobalamin (B12) | 0.898 |
| Ascorbic Acid (C) | 3.347 |
| Linoleic Acid (F) | 0.276 |
| Tocopherol (E) | 0.007 |
| Menadione (K3) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño-Ortiz, J.; Redondo-Calvo, F.J.; Lozano-Rodríguez, R.; Terrón-Arcos, V.; Bergón-Gutiérrez, M.; Rodríguez-Jiménez, C.; Rodríguez, J.F.; del Campo, R.; Gómez, L.A.; Bejarano-Ramírez, N.; et al. Thiosulfinate-Enriched Allium sativum Extract Exhibits Differential Effects between Healthy and Sepsis Patients: The Implication of HIF-1α. Int. J. Mol. Sci. 2023, 24, 6234. https://doi.org/10.3390/ijms24076234
Avendaño-Ortiz J, Redondo-Calvo FJ, Lozano-Rodríguez R, Terrón-Arcos V, Bergón-Gutiérrez M, Rodríguez-Jiménez C, Rodríguez JF, del Campo R, Gómez LA, Bejarano-Ramírez N, et al. Thiosulfinate-Enriched Allium sativum Extract Exhibits Differential Effects between Healthy and Sepsis Patients: The Implication of HIF-1α. International Journal of Molecular Sciences. 2023; 24(7):6234. https://doi.org/10.3390/ijms24076234
Chicago/Turabian StyleAvendaño-Ortiz, José, Francisco Javier Redondo-Calvo, Roberto Lozano-Rodríguez, Verónica Terrón-Arcos, Marta Bergón-Gutiérrez, Concepción Rodríguez-Jiménez, Juan Francisco Rodríguez, Rosa del Campo, Luis Antonio Gómez, Natalia Bejarano-Ramírez, and et al. 2023. "Thiosulfinate-Enriched Allium sativum Extract Exhibits Differential Effects between Healthy and Sepsis Patients: The Implication of HIF-1α" International Journal of Molecular Sciences 24, no. 7: 6234. https://doi.org/10.3390/ijms24076234