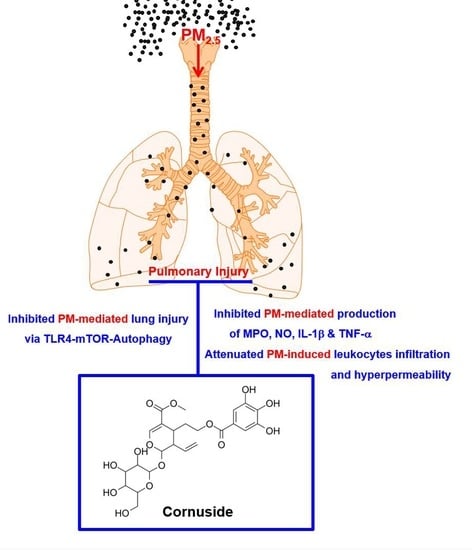
Therapeutic Effects of Cornuside on Particulate Matter–Induced Lung Injury
Abstract
:1. Introduction
2. Results
2.1. Effects of CN Lung Damage by PM2.5
2.2. Effects of CN on Vascular Barrier Disruptive Responses by PM2.5
2.3. Effects of CN on Pulmonary Inflammatory Responses by PM2.5
2.4. Effects of CN on Signaling Pathways by PM2.5
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Care
4.3. Wet/Dry Weight Ratio of the Lung Tissue
4.4. Culture of Mouse Lung Microvascular Endothelial Cell (MLMVECs) and siRNA Transfection
4.5. Hematoxylin and Eosin Staining
4.6. Enzyme-Linked Immunosorbent Assay of p38, Mitogen-Activated Protein Kinase, Myeloperoxidase, Nitrous Oxide, Interleukon-1β, and TNF-α Phosphorylation
4.7. Protein Concentration and Cell Count in the BALF
4.8. Permeability Assays
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [PubMed]
- Cho, C.C.; Hsieh, W.Y.; Tsai, C.H.; Chen, C.Y.; Chang, H.F.; Lin, C.S. In Vitro and In Vivo Experimental Studies of PM2.5 on Disease Progression. Int. J. Environ. Res. Public Health 2018, 15, 1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, J.J.; Li, J.; Yang, T.J.; Cui, J.; Xia, X.; Chen, G.P.; Zheng, S.Y.; Bao, J.H.; Wang, T.; Shen, M.L.; et al. Long-term exposure to air pollution and lung function among children in China: Association and effect modification. Front. Public Health 2022, 10, 988242. [Google Scholar] [CrossRef]
- Cao, L.; Lin, H.; Li, Q.; Han, S.; Yin, H.; Zhang, N.; Gao, Y.; Chen, Y.; Ping, F. Study on Lung Injury Caused by Fine Particulate Matter and Intervention Effect of Rhodiola wallichiana. Evid.-Based Compl. Alt. 2022, 2022, 3693231. [Google Scholar] [CrossRef]
- Wang, N.; Mengersen, K.; Kimlin, M.; Zhou, M.; Tong, S.; Fang, L.; Wang, B.; Hu, W. Lung cancer and particulate pollution: A critical review of spatial and temporal analysis evidence. Environ. Res. 2018, 164, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, J.; Liu, J.; Zheng, X.; Yang, D.; Lu, M. Toll-like receptor-mediated innate immunity orchestrates adaptive immune responses in HBV infection. Front. Immunol. 2022, 13, 965018. [Google Scholar] [CrossRef]
- Nagappan, A.; Park, S.B.; Lee, S.J.; Moon, Y. Mechanistic Implications of Biomass-Derived Particulate Matter for Immunity and Immune Disorders. Toxics 2021, 9, 18. [Google Scholar] [CrossRef]
- Yan, J.; Xie, Y.; Si, J.; Gan, L.; Li, H.; Sun, C.; Di, C.; Zhang, J.; Huang, G.; Zhang, X.; et al. Crosstalk of the Caspase Family and Mammalian Target of Rapamycin Signaling. Int. J. Mol. Sci. 2021, 22, 817. [Google Scholar] [CrossRef]
- Gao, Y.H.; Wang, C.S.; Jiang, D.; An, G.; Jin, F.; Zhang, J.C.; Han, G.K.; Cui, C.M.; Jiang, P. New insights into the interplay between autophagy and oxidative and endoplasmic reticulum stress in neuronal cell death and survival. Front. Cell Dev. Biol. 2022, 10, 994037. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Fu, Z. Role of autophagy in lung diseases and ageing. Eur. Respir. Rev. 2022, 31, 220134. [Google Scholar] [CrossRef]
- Kang, D.G.; Moon, M.K.; Lee, A.S.; Kwon, T.O.; Kim, J.S.; Lee, H.S. Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol. Pharm. Bull. 2007, 30, 1796–1799. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.L.; Chen, X.G.; Zhu, H.B.; Hou, J.; Tian, J.W. Cornuside attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Pharmacology 2009, 84, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, M.T.; Liu, Q.; Lamorie-Foote, K.; Shkirkova, K.; Connor, M.; Patel, A.; Montagne, A.; Baertsch, H.; Sioutas, C.; Morgan, T.E.; et al. Air Pollution Particulate Matter Amplifies White Matter Vascular Pathology and Demyelination Caused by Hypoperfusion. Front. Immunol. 2021, 12, 785519. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wen, Q.; Zhang, R. Sources, health effects and control strategies of indoor fine particulate matter (PM2.5): A review. Sci. Total Environ. 2017, 586, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.W.; Lee, T.L.; Chen, Y.C.; Liang, C.J.; Wang, S.H.; Lue, J.H.; Tsai, J.S.; Lee, S.W.; Chen, S.H.; Yang, Y.F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-kappaB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Qiu, X.; Hu, X.; Shang, Y.; Pardo, M.; Fang, Y.; Wang, J.; Rudich, Y.; Zhu, T. Effects on IL-1beta signaling activation induced by water and organic extracts of fine particulate matter (PM2.5) in vitro. Environ. Pollut. 2018, 237, 592–600. [Google Scholar] [CrossRef]
- Blondonnet, R.; Constantin, J.M.; Sapin, V.; Jabaudon, M. A Pathophysiologic Approach to Biomarkers in Acute Respiratory Distress Syndrome. Dis. Markers 2016, 2016, 3501373. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.Z.; Sun, H.; Chen, J.H. Histone deacetylases 3 deletion restrains PM2.5-induced mice lung injury by regulating NF-kappaB and TGF-beta/Smad2/3 signaling pathways. Biomed. Pharmacother. 2017, 85, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shi, Q.; Zhang, L.; Zhao, H. High molecular weight hyaluronan attenuates fine particulate matter-induced acute lung injury through inhibition of ROS-ASK1-p38/JNK-mediated epithelial apoptosis. Environ. Toxicol. Pharmacol. 2018, 59, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, X.; Cao, L.; Shen, Z.X.; Lei, Y.; Cao, Y.X. Airborne fine particulate matter induces an upregulation of endothelin receptors on rat bronchi. Environ. Pollut. 2016, 209, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, W.; Kim, E.; Ku, S.K.; Bae, J.S. Inhibitory effects of collismycin C and pyrisulfoxin A on particulate matter-induced pulmonary injury. Phytomedicine 2019, 62, 152939. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, Q.; Zhang, X.; Wu, S.; Wang, S.; Chen, R.; Li, X. Role of astrocyte activation in fine particulate matter-enhancement of existing ischemic stroke in Sprague-Dawley male rats. J. Toxicol. Environ. Health A 2016, 79, 393–401. [Google Scholar] [CrossRef]
- Yan, X.D.; Wang, Q.M.; Tie, C.; Jin, H.T.; Han, Y.X.; Zhang, J.L.; Yu, X.M.; Hou, Q.; Zhang, P.P.; Wang, A.P.; et al. Polydatin protects the respiratory system from PM2.5 exposure. Sci. Rep. 2017, 7, 40030. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Fujishima, K.; Yatera, K.; Yamamoto, K. Usefulness of Intratracheal Instillation Studies for Estimating Nanoparticle-Induced Pulmonary Toxicity. Int. J. Mol. Sci. 2016, 17, 165. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Huang, Q.; Deng, S.; Yang, Y.; Li, J.; Wang, S. Mechanisms of TLR4-Mediated Autophagy and Nitroxidative Stress. Front. Cell Infect. Microbiol. 2021, 11, 766590. [Google Scholar] [CrossRef]
- Woodward, N.C.; Levine, M.C.; Haghani, A.; Shirmohammadi, F.; Saffari, A.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J. Neuroinflamm. 2017, 14, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675. [Google Scholar] [CrossRef]
- Hu, Y.; Lou, J.; Mao, Y.Y.; Lai, T.W.; Liu, L.Y.; Zhu, C.; Zhang, C.; Liu, J.; Li, Y.Y.; Zhang, F.; et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 2016, 12, 2286–2299. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Lai, D.; Zhang, L.; Xu, H. Induction of Autophagy and Apoptosis via PI3K/AKT/TOR Pathways by Azadirachtin A in Spodoptera litura Cells. Sci. Rep. 2016, 6, 35482. [Google Scholar] [CrossRef] [Green Version]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018, 6, 32. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergvall, C.; Westerholm, R. Determination of dibenzopyrenes in standard reference materials (SRM) 1649a, 1650, and 2975 using ultrasonically assisted extraction and LC-GC-MS. Anal. Bioanal. Chem. 2006, 384, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ryu, S.H.; Choi, H.; Park, D.H.; Bae, J.S. The Inhibitory Functions of Sparstolonin B against Ambient Fine Particulate Matter Induced Lung Injury. Biotechnol. Bioprocess Eng. 2022, 27, 949–960. [Google Scholar] [CrossRef]
- Sim, H.; Noh, Y.; Choo, S.; Kim, N.; Lee, T.; Bae, J.S. Suppressive Activities of Fisetin on Particulate Matter-induced Oxidative Stress. Biotechnol. Bioprocess Eng. 2021, 26, 568–574. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, G.E.; Song, G.Y.; Bae, J.S. Pulmonary protective functions of rare ginsenoside Rg4 on particulate matter-induced inflammatory responses. Biotechnol. Bioprocess Eng. 2019, 24, 445–453. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, W.; Yang, S.; Cho, S.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive effects of rare ginsenosides, Rk1 and Rg5, on HMGB1-mediated septic responses. Food Chem. Toxicol. 2019, 124, 45–53. [Google Scholar] [CrossRef]
- Lee, I.C.; Bae, J.S. Pelargonidin Protects against Renal Injury in a Mouse Model of Sepsis. J. Med. Food 2019, 22, 57–61. [Google Scholar] [CrossRef]
- Lee, W.; Cho, S.H.; Kim, J.E.; Lee, C.; Lee, J.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive Effects of Ginsenoside Rh1 on HMGB1-Mediated Septic Responses. Am. J. Chin. Med. 2019, 47, 119–133. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.C. Growth Inhibitory Effect of Mangiferin on Thyroid Cancer Cell Line TPC1. Biotechnol. Bioprocess Eng. 2018, 23, 649–654. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.O.; Park, E.K.; Park, D.H.; Song, G.Y.; Bae, J.-S. Therapeutic Effects of Cornuside on Particulate Matter–Induced Lung Injury. Int. J. Mol. Sci. 2023, 24, 4979. https://doi.org/10.3390/ijms24054979
Kim GO, Park EK, Park DH, Song GY, Bae J-S. Therapeutic Effects of Cornuside on Particulate Matter–Induced Lung Injury. International Journal of Molecular Sciences. 2023; 24(5):4979. https://doi.org/10.3390/ijms24054979
Chicago/Turabian StyleKim, Go Oun, Eui Kyun Park, Dong Ho Park, Gyu Yong Song, and Jong-Sup Bae. 2023. "Therapeutic Effects of Cornuside on Particulate Matter–Induced Lung Injury" International Journal of Molecular Sciences 24, no. 5: 4979. https://doi.org/10.3390/ijms24054979