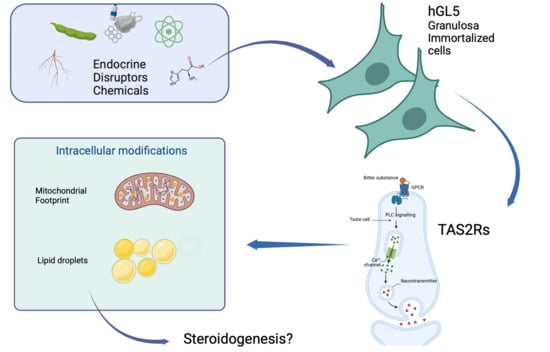
Bitter Taste Receptors and Endocrine Disruptors: Cellular and Molecular Insights from an In Vitro Model of Human Granulosa Cells
Abstract
:1. Introduction
2. Results
2.1. hGL5 Express Bitter Taste Receptors
2.2. Effect of EDCs Exposure on Viability of hGL5 Cells
2.3. EDCs Acts as Agonists on TAS2Rs Affecting Mitochondrial Footprint
2.4. BCA and Caffeine Affect TAS2Rs Relative Abundance
2.5. BCA and Caffeine Affect Intracellular Lipid Storage and Steroid Secretion
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. hGL5 Cell Line
4.3. Cell Proliferation
4.4. Compounds
4.5. Cytotoxicity Assay
4.6. Mitotracker
4.7. RNA Extraction Complementary DNA Preparation
4.8. qPCR
4.9. Western Blot Analysis
4.10. Oil Red O (ORO) Staining and Morphological Observation
4.11. Transmission Electron Microscopy
ELISA Test
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine Disrupting Chemicals: Impacts on Human Fertility and Fecundity during the Peri-Conception Period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Gaskins, A.J. Female Exposure to Endocrine Disrupting Chemicals and Fecundity: A Review. Curr. Opin. Obstet. Gynecol. 2017, 29, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor Beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Plunk, E.C.; Richards, S.M. Epigenetic Modifications Due to Environment, Ageing, Nutrition, and Endocrine Disrupting Chemicals and Their Effects on the Endocrine System. Int. J. Endocrinol. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Collotta, M.; Bertazzi, P.A.; Bollati, V. Epigenetics and Pesticides. Toxicology 2013, 307, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.K.T.; O’Donnell, A.H.; Bestor, T.H. Mammalian Cytosine Methylation at a Glance. J. Cell Sci. 2009, 122, 2787–2791. [Google Scholar] [CrossRef] [Green Version]
- Brachova, P.; Hung, W.-T.; McGinnis, L.K.; Christenson, L.K. MicroRNA Regulation of Endocrine Functions in the Ovary. In Post-Transcriptional Mechanisms in Endocrine Regulation; Menon, K.M.J., Goldstrohm, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 109–127. ISBN 978-3-319-25122-6. [Google Scholar]
- Carletti, M.Z.; Fiedler, S.D.; Christenson, L.K. MicroRNA 21 Blocks Apoptosis in Mouse Periovulatory Granulosa Cells1. Biol. Reprod. 2010, 83, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Sabry, R.; Saleh, A.C.; Stalker, L.; LaMarre, J.; Favetta, L.A. Effects of Bisphenol A and Bisphenol S on MicroRNA Expression during Bovine (Bos Taurus) Oocyte Maturation and Early Embryo Development. Reprod. Toxicol. 2021, 99, 96–108. [Google Scholar] [CrossRef]
- Rodosthenous, R.S.; Baccarelli, A.A.; Mansour, A.; Adir, M.; Israel, A.; Racowsky, C.; Hauser, R.; Bollati, V.; Machtinger, R. Supraphysiological Concentrations of Bisphenol A Alter the Expression of Extracellular Vesicle-Enriched MiRNAs From Human Primary Granulosa Cells. Toxicol. Sci. 2019, 169, 5–13. [Google Scholar] [CrossRef]
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho, S.; Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J.; et al. Female Reproductive Disorders: The Roles of Endocrine-Disrupting Compounds and Developmental Timing. Fertil. Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, V.R.; Rumph, J.T.; Ameli, S.; Bruner-Tran, K.L.; Osteen, K.G. The Potential Relationship Between Environmental Endocrine Disruptor Exposure and the Development of Endometriosis and Adenomyosis. Front. Physiol. 2022, 12, 807685. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Vincken, J.-P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors HTAS2R14 and HTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Wang, W.C.H.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.K.; Liggett, S.B. Bitter Taste Receptors on Airway Smooth Muscle Bronchodilate by Localized Calcium Signaling and Reverse Obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, K. Expression of the Sweet Receptor Protein, T1R3, in the Human Liver and Pancreas. J. Vet. Med. Sci. 2004, 66, 1311–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, R.A.; Kapoor, S.; Tincello, D.G. Expression and Distribution of the Sweet Taste Receptor Isoforms T1R2 and T1R3 in Human and Rat Bladders. J. Urol. 2011, 186, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Voigt, A.; Hübner, S.; Döring, L.; Perlach, N.; Hermans-Borgmeyer, I.; Boehm, U.; Meyerhof, W. Cre-Mediated Recombination in Tas2r131 Cells—A Unique Way to Explore Bitter Taste Receptor Function Inside and Outside of the Taste System. Chem. Senses 2015, 40, 627–639. [Google Scholar] [CrossRef] [Green Version]
- Luddi, A.; Governini, L.; Wilmskötter, D.; Gudermann, T.; Boekhoff, I.; Piomboni, P. Taste Receptors: New Players in Sperm Biology. Int. J. Mol. Sci. 2019, 20, 967. [Google Scholar] [CrossRef] [Green Version]
- Fehr, J.; Meyer, D.; Widmayer, P.; Borth, H.C.; Ackermann, F.; Wilhelm, B.; Gudermann, T.; Boekhoff, I. Expression of the G-Protein α-Subunit Gustducin in Mammalian Spermatozoa. J. Comp. Physiol. A 2007, 193, 21–34. [Google Scholar] [CrossRef]
- Gilca, M.; Dragos, D. Extraoral Taste Receptor Discovery: New Light on Ayurvedic Pharmacology. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–30. [Google Scholar] [CrossRef]
- Governini, L.; Semplici, B.; Pavone, V.; Crifasi, L.; Marrocco, C.; De Leo, V.; Arlt, E.; Gudermann, T.; Boekhoff, I.; Luddi, A.; et al. Expression of Taste Receptor 2 Subtypes in Human Testis and Sperm. J. Clin. Med. 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeruzal-Świątecka, J.; Fendler, W.; Pietruszewska, W. Clinical Role of Extraoral Bitter Taste Receptors. Int. J. Mol. Sci. 2020, 21, 5156. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-Secreted Factors: Regulators of Cumulus Cell Function and Oocyte Quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainey, W.H.; Sawetawan, C.; Shay, J.W.; Michael, M.D.; Mathis, J.M.; Kutteh, W.; Byrd, W.; Carr, B.R. Transformation of Human Granulosa Cells with the E6 and E7 Regions of Human Papillomavirus. J. Clin. Endocrinol. Metab. 1994, 78, 705–710. [Google Scholar] [CrossRef]
- Casarini, L.; Reiter, E.; Simoni, M. β-Arrestins Regulate Gonadotropin Receptor-Mediated Cell Proliferation and Apoptosis by Controlling Different FSHR or LHCGR Intracellular Signaling in the HGL5 Cell Line. Mol. Cell. Endocrinol. 2016, 437, 11–21. [Google Scholar] [CrossRef]
- Casarini, L.; Riccetti, L.; De Pascali, F.; Gilioli, L.; Marino, M.; Vecchi, E.; Morini, D.; Nicoli, A.; La Sala, G.; Simoni, M. Estrogen Modulates Specific Life and Death Signals Induced by LH and HCG in Human Primary Granulosa Cells In Vitro. Int. J. Mol. Sci. 2017, 18, 926. [Google Scholar] [CrossRef] [Green Version]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Sharma, P.; Yi, R.; Nayak, A.P.; Wang, N.; Tang, F.; Knight, M.J.; Pan, S.; Oliver, B.; Deshpande, D.A. Bitter Taste Receptor Agonists Mitigate Features of Allergic Asthma in Mice. Sci. Rep. 2017, 7, 46166. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A Simple ImageJ Macro Tool for Analyzing Mitochondrial Network Morphology in Mammalian Cell Culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Gore, A.C.; Crews, D. Environmental Endocrine Disruption of Brain and Behavior. In Hormones, Brain and Behavior; Elsevier: Amsterdam, The Netherlands, 2017; pp. 63–88. ISBN 978-0-12-803608-2. [Google Scholar]
- Wong, C.K.; Keung, W.M. Bovine Adrenal 3β-Hydroxysteroid Dehydrogenase (E.C. 1.1.1.145)/5-Ene-4-Ene Isomerase (E.C. 5.3.3.1): Characterization and Its Inhibition by Isoflavones. J. Steroid Biochem. Mol. Biol. 1999, 71, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Almstrup, K.; Fernández, M.F.; Petersen, J.H.; Olea, N.; Skakkebaek, N.E.; Leffers, H. Dual Effects of Phytoestrogens Result in U-Shaped Dose-Response Curves. Environ. Health Perspect. 2002, 110, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordhoff, V.; Sonntag, B.; von Tils, D.; Götte, M.; Schüring, A.N.; Gromoll, J.; Redmann, K.; Casarini, L.; Simoni, M. Effects of the FSH Receptor Gene Polymorphism p.N680S on CAMP and Steroid Production in Cultured Primary Human Granulosa Cells. Reprod. Biomed. Online 2011, 23, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havelock, J.C.; Rainey, W.E.; Carr, B.R. Ovarian Granulosa Cell Lines. Mol. Cell. Endocrinol. 2004, 228, 67–78. [Google Scholar] [CrossRef]
- Semplici, B.; Luongo, F.P.; Passaponti, S.; Landi, C.; Governini, L.; Morgante, G.; De Leo, V.; Piomboni, P.; Luddi, A. Bitter Taste Receptors Expression in Human Granulosa and Cumulus Cells: New Perspectives in Female Fertility. Cells 2021, 10, 3127. [Google Scholar] [CrossRef]
- Gravina, S.A.; Yep, G.L.; Khan, M. Human Biology of Taste. Ann. Saudi Med. 2013, 33, 217–222. [Google Scholar] [CrossRef]
- Henry, L.A.; Witt, D.M. Resveratrol: Phytoestrogen Effects on Reproductive Physiology and Behavior in Female Rats. Horm. Behav. 2002, 41, 220–228. [Google Scholar] [CrossRef]
- Sreerangaraja Urs, D.B.; Wu, W.-H.; Komrskova, K.; Postlerova, P.; Lin, Y.-F.; Tzeng, C.-R.; Kao, S.-H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef]
- Bassi, G.; Sidhu, S.K.; Mishra, S. The Expanding Role of Mitochondria, Autophagy and Lipophagy in Steroidogenesis. Cells 2021, 10, 1851. [Google Scholar] [CrossRef]
- Duarte, A.; Poderoso, C.; Cooke, M.; Soria, G.; Cornejo Maciel, F.; Gottifredi, V.; Podestá, E.J. Mitochondrial Fusion Is Essential for Steroid Biosynthesis. PLoS ONE 2012, 7, e45829. [Google Scholar] [CrossRef]
- Park, J.-E.; Kim, Y.-J.; Lee, S.G.; Kim, J.Y.; Chung, J.-Y.; Jeong, S.-Y.; Koh, H.; Yun, J.; Park, H.T.; Yoo, Y.H.; et al. Drp1 Phosphorylation Is Indispensable for Steroidogenesis in Leydig Cells. Endocrinology 2019, 160, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-S.; Chang, H.-Y.; Kao, S.-H.; Kao, C.-H.; Wu, Y.-C.; Yeh, S.; Tzeng, C.-R.; Chang, C. Abnormal Mitochondrial Function and Impaired Granulosa Cell Differentiation in Androgen Receptor Knockout Mice. Int. J. Mol. Sci. 2015, 16, 9831–9849. [Google Scholar] [CrossRef] [PubMed]
- Nynca, A.; Swigonska, S.; Piasecka, J.; Kolomycka, A.; Kaminska, B.; Radziewicz-Pigiel, M.; Gut-Nagel, M.; Ciereszko, R.E. Biochanin A Affects Steroidogenesis and Estrogen Receptor-β Expression in Porcine Granulosa Cells. Theriogenology 2013, 80, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Vats, R.; Sawhney, A.K. Changes in Follicular Lipids during Follicular Growth in the Goat (Capra Hircus) Ovary. Small Rumin. Res. 1996, 20, 177–180. [Google Scholar] [CrossRef]
- Gao, S.; Gan, X.; He, H.; Hu, S.; Deng, Y.; Chen, X.; Li, L.; Hu, J.; Li, L.; Wang, J. Dynamic Characteristics of Lipid Metabolism in Cultured Granulosa Cells from Geese Follicles at Different Developmental Stages. Biosci. Rep. 2019, 39, BSR20192188. [Google Scholar] [CrossRef]
- Governini, L.; Carrarelli, P.; Rocha, A.L.L.; Leo, V.D.; Luddi, A.; Arcuri, F.; Piomboni, P.; Chapron, C.; Bilezikjian, L.M.; Petraglia, F. FOXL2 in Human Endometrium: Hyperexpressed in Endometriosis. Reprod. Sci. 2014, 21, 1249–1255. [Google Scholar] [CrossRef]
- Focarelli, R.; Luddi, A.; De Leo, V.; Capaldo, A.; Stendardi, A.; Pavone, V.; Benincasa, L.; Belmonte, G.; Petraglia, F.; Piomboni, P. Dysregulation of GdA Expression in Endometrium of Women With Endometriosis: Implication for Endometrial Receptivity. Reprod. Sci. 2018, 25, 579–586. [Google Scholar] [CrossRef]
- Puggioni, E.; Governini, L.; Gori, M.; Belmonte, G.; Piomboni, P.; Costantino-Ceccarini, E.; Luddi, A. Morphological and Molecular Characterisation of Twitcher Mouse Spermatogenesis: An Update. Reprod. Fertil. Dev. 2016, 28, 1258. [Google Scholar] [CrossRef]
- Luongo, F.P.; Dragoni, F.; Boccuto, A.; Paccagnini, E.; Gentile, M.; Canosi, T.; Morgante, G.; Luddi, A.; Zazzi, M.; Vicenti, I.; et al. SARS-CoV-2 Infection of Human Ovarian Cells: A Potential Negative Impact on Female Fertility. Cells 2022, 11, 1431. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luongo, F.P.; Passaponti, S.; Haxhiu, A.; Raeispour, M.; Belmonte, G.; Governini, L.; Casarini, L.; Piomboni, P.; Luddi, A. Bitter Taste Receptors and Endocrine Disruptors: Cellular and Molecular Insights from an In Vitro Model of Human Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 15540. https://doi.org/10.3390/ijms232415540
Luongo FP, Passaponti S, Haxhiu A, Raeispour M, Belmonte G, Governini L, Casarini L, Piomboni P, Luddi A. Bitter Taste Receptors and Endocrine Disruptors: Cellular and Molecular Insights from an In Vitro Model of Human Granulosa Cells. International Journal of Molecular Sciences. 2022; 23(24):15540. https://doi.org/10.3390/ijms232415540
Chicago/Turabian StyleLuongo, Francesca Paola, Sofia Passaponti, Alesandro Haxhiu, Maryam Raeispour, Giuseppe Belmonte, Laura Governini, Livio Casarini, Paola Piomboni, and Alice Luddi. 2022. "Bitter Taste Receptors and Endocrine Disruptors: Cellular and Molecular Insights from an In Vitro Model of Human Granulosa Cells" International Journal of Molecular Sciences 23, no. 24: 15540. https://doi.org/10.3390/ijms232415540