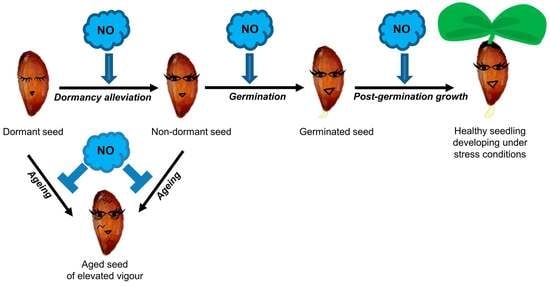
Nitric Oxide in Seed Biology
Abstract
:1. Introduction
1.1. Historical View of NO Plant-Related Literature
1.2. NO Sources in Seeds and NO Donors Used in Laboratory Practice
2. Seed Priming or Presoaking with SNP Used as NO Donor Increases Seed Germination and Seedling Tolerance to Various Stresses
3. NO-Phytohormone Network in Seed Dormancy and Germination
4. NO–ROS Link in the Regulation of Seed Biology
5. Maintenance of NO Balance and NO-Dependent Modifications in the Regulation of Seed Physiology
6. NO as a Seed Antiaging Molecule
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fang, P.; Sun, T.; Wang, Y.; Ding, Y.; Pandey, A.K.; Zhu, C.; Xu, P. Plant Gasotransmitters: Light Molecules Interplaying with Heavy Metals. Rev. Env. Sci. Biotechnol. 2021, 20, 31–53. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric Oxide and Hydrogen Sulfide in Plants: Which Comes First? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Sparrman, B.; Ehrenberg, L.; Ehrenberg, A.; Andersson, G.; Stenhagen, E.; Palmstierna, H. Scavenging of Free Radicals and Radiation Protection by Nitric Oxide in Plant Seeds. Acta. Chem. Scand. 1959, 13, 199–200. [Google Scholar] [CrossRef]
- Klepper, L. Nitric Oxide (NO) and Nitrogen Dioxide (NO2) Emissions from Herbicide-Treated Soybean Plants. Atmos. Environ. 1979, 13, 537–542. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric Oxide Functions as a Signal in Plant Disease Resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Giba, Z.; Grubišić, D.; Todorović, S.; Sajc, L.; Stojaković, D.; Konjević, R. Effect of Nitric Oxide–Releasing Compounds on Phytochrome–Controlled Germination of Empress Tree Seeds. Plant Growth Regul. 1998, 26, 175–181. [Google Scholar] [CrossRef]
- Grubišić, D.; Konjević, R. Light and Nitrate Interaction in Phytochrome-Controlled Germination of Paulownia Tomentosa Seeds. Planta 1990, 181, 239–243. [Google Scholar] [CrossRef]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a Signal Relieving Seed Dormancy in Arabidopsis. Plant Cell Env. 2005, 28, 500–512. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J. Trace Gas Emissions and Smoke-Induced Seed Germination. Science 1997, 276, 1248–1250. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S. Nitric Oxide: A Key Driver of Signaling in Plants. MOJ Ecol. Environ. Sci. 2018, 3, 1. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitric Oxide Synthase-like Activity in Higher Plants. Nitric Oxide 2017, 68, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Mounier, A.; Santolini, J.; Jeandroz, S.; Wendehenne, D.; Brouquisse, R. The Evolution of Nitric Oxide Signalling Diverges between Animal and Green Lineages. J. Exp. Bot. 2019, 70, 4355–4364. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Kaladhar, V.C.; Fitzpatrick, T.B.; Fernie, A.R.; Møller, I.M.; Loake, G.J. Nitric Oxide Regulation of Plant Metabolism. Mol. Plant 2022, 15, 228–242. [Google Scholar] [CrossRef]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic Synthesis of Nitric Oxide by Plant Tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Simontacchi, M.; Jasid, S.; Puntarulo, S. Nitric Oxide Generation during Early Germination of Sorghum Seeds. Plant Sci. 2004, 167, 839–847. [Google Scholar] [CrossRef]
- Gupta, K.J.; Igamberdiev, A.U.; Manjunatha, G.; Segu, S.; Moran, J.F.; Neelawarne, B.; Bauwe, H.; Kaiser, W.M. The Emerging Roles of Nitric Oxide (NO) in Plant Mitochondria. Plant Sci. 2011, 181, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Bykova, N.V.; Hu, J.; Ma, Z.; Igamberdiev, A.U. The Role of Reactive Oxygen and Nitrogen Species in Bioenergetics, Metabolism, and Signaling during Seed Germination. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Signaling and Communication in Plants; Gupta, K.J., Igamberdiev, A.U., Eds.; Springer: Cham, Switzerland, 2015; pp. 177–195. [Google Scholar]
- Ciacka, K.; Krasuska, U.; Staszek, P.; Wal, A.; Zak, J.; Gniazdowska, A. Effect of Nitrogen Reactive Compounds on Aging in Seed. Front. Plant Sci. 2020, 11, 1011. [Google Scholar] [CrossRef]
- Gupta, K.J.; Hancock, J.T.; Petrivalsky, M.; Kolbert, Z.; Lindermayr, C.; Durner, J.; Barroso, J.B.; Palma, J.M.; Brouquisse, R.; Wendehenne, D.; et al. Recommendations on Terminology and Experimental Best Practice Associated with Plant Nitric Oxide Research. New Phytol. 2020, 225, 1828–1834. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kosmala, A. Are Nitric Oxide Donors a Valuable Tool to Study the Functional Role of Nitric Oxide in Plant Metabolism? Plant Biol. 2011, 13, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, T.; Oberle, S.; Abate, A.; Schröder, H. Photoactivation of the Nitric Oxide Donor SIN-1. FEBS Lett. 1997, 406, 66–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, J.N.; Baker, M.T.; Guerra, R.; Harrison, D.G. Nitric Oxide Generation from Nitroprusside by Vascular Tissue: Evidence That Reduction of the Nitroprusside Anion and Cyanide Loss Are Required. Biochem. Pharm. 1991, 42, S157–S165. [Google Scholar] [CrossRef] [PubMed]
- Shishido, S.M.; de Oliveira, M.G. Photosensitivity of Aqueous Sodium Nitroprusside Solutions: Nitric Oxide Release versus Cyanide Toxicity. Prog. React. Kinet. Mech. 2001, 26, 239–261. [Google Scholar] [CrossRef]
- Huerta, S.; Chilka, S.; Bonavida, B. Nitric Oxide Donors: Novel Cancer Therapeutics (Review). Int. J. Oncol. 2008, 33, 909–927. [Google Scholar] [CrossRef] [Green Version]
- Melvin, A.C.; Jones, W.M.; Lutzke, A.; Allison, C.L.; Reynolds, M.M. S-Nitrosoglutathione Exhibits Greater Stability than S-Nitroso-N-Acetylpenicillamine under Common Laboratory Conditions: A Comparative Stability Study. Nitric Oxide 2019, 92, 18–25. [Google Scholar] [CrossRef]
- Singh, R.J.; Hogg, N.; Joseph, J.; Konorev, E.; Kalyanaraman, B. The Peroxynitrite Generator, SIN-1, Becomes a Nitric Oxide Donor in the Presence of Electron Acceptors. Arch. Biochem. Biophys. 1999, 361, 331–339. [Google Scholar] [CrossRef]
- Keefer, L.K.; Nims, R.W.; Davies, K.M.; Wink, D.A. “NONOates” (1-Substituted Diazen-1-Ium-1,2-Diolates) as Nitric Oxide Donors: Convenient Nitric Oxide Dosage Forms. Methods Enzym. 1996, 268, 281–293. [Google Scholar] [CrossRef]
- Li, B.; Ming, Y.; Liu, Y.; Xing, H.; Fu, R.; Li, Z.; Ni, R.; Li, L.; Duan, D.; Xu, J.; et al. Recent Developments in Pharmacological Effect, Mechanism and Application Prospect of Diazeniumdiolates. Front. Pharm. 2020, 11, 923. [Google Scholar] [CrossRef]
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and Nitrate Chemical Biology and Signalling. Br. J. Pharm. 2019, 176, 228. [Google Scholar] [CrossRef]
- Yamasaki, H. Nitrite-Dependent Nitric Oxide Production Pathway: Implications for Involvement of Active Nitrogen Species in Photoinhibition in Vivo. Philos. Trans. R Soc. Lond. B Biol. Sci. 2000, 355, 1477–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janczyk, A.; Wolnicka-Glubisz, A.; Chmura, A.; Elas, M.; Matuszak, Z.; Stochel, G.; Urbanska, K. NO-Dependent Phototoxicity of Roussin’s Black Salt against Cancer Cells. Nitric Oxide 2004, 10, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Zhao, S.; Arif, S.; Zhao, Y.; Ming, L.C.; Huang, D. Seed Priming Technology as a Key Strategy to Increase Crop Plant Production under Adverse Environmental Conditions. J. Agric. Hortic. Res. 2022, 5, 27–46. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology-Basic and Translational Research Driving Seed Technology; Araujo, S., Balestrazzi, A., Eds.; IntechOpen: London, UK, 2016; pp. 1–46. ISBN 978-953-51-2659-1. [Google Scholar]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric Oxide Function in Plant Abiotic Stress. Plant Cell Env. 2017, 40, 462–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S. Nitric Oxide-Mediated Integrative Alterations in Plant Metabolism to Confer Abiotic Stress Tolerance, NO Crosstalk with Phytohormones and NO-Mediated Post Translational Modifications in Modulating Diverse Plant Stress. Nitric Oxide 2018, 73, 22–38. [Google Scholar] [CrossRef]
- Yadav, A.; Mathan, J.; Bhati, K.K.; Singh, A. Nitric Oxide Signaling and Abiotic Stress Tolerance in Plants. In Nitric Oxide in Plant Biology: An Ancient Molecule with Emerging Roles; Singh, V.P., Singh, S., Tripathi, D.K., Romero-Puertas, M.C., Sandalio, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 373–390. ISBN 9780128187975. [Google Scholar]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Haider, M.Z.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum Aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Karimi, Z.; Khara, J.; Habibi, G. Combined Hydrogen Peroxide and Nitric Oxide Priming Modulate Salt Stress Tolerance in Acclimated and Non-Acclimated Oilseed Rape (Brassica Napus L.) Plants. J. Plant Physiol. Breed. 2020, 10, 27–43. [Google Scholar] [CrossRef]
- Flórez, M.; Carbonell, M.V.; Martínez, E. Exposure of Maize Seeds to Stationary Magnetic Fields: Effects on Germination and Early Growth. Env. Exp. Bot. 2007, 59, 68–75. [Google Scholar] [CrossRef]
- Patel, P.; Kadur Narayanaswamy, G.; Kataria, S.; Baghel, L. Involvement of Nitric Oxide in Enhanced Germination and Seedling Growth of Magnetoprimed Maize Seeds. Plant Signal Behav. 2017, 12, e1293217. [Google Scholar] [CrossRef] [Green Version]
- Bibi, A.; Majid, S.A.; Azhar, N.; Amjad, M.S.; Ashraf, S.; Ahmad, I.; Mumtaz, S.; Ijaz, S. Differential Changes in Growth and Enzyme Activities of Chilling Induced Wheat Seedlings by Nitric Oxide Priming. Int. J. Agric. Biol. 2020, 23, 919–926. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of Seed Priming Treatments on the Germination and Development of Two Rapeseed (Brassica Napus L.) Varieties under the Co-Influence of Low Temperature and Drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef] [PubMed]
- Gama, G.; Cunha, P.; Fialho, C.; Pinheiro, D.; Paula, I. Effect of Nitric Oxide on Seed Germination and Seedlings Development of Carrot under Water Deficit. J. Exp. Agric. Int. 2018, 22, 1–7. [Google Scholar] [CrossRef]
- Zhang, H. Nitric Oxide Alleviates the Inhibition of Salinity Stress on Seed Germination and Seedling Growth of Cynanchum Bungei Decne (Asclepiadaceae). HortScience 2015, 50, 119–122. [Google Scholar] [CrossRef]
- Shams, M.; Yildirim, E.; Agar, G.; Ercisli, S.; Ekinci, M.; Dursun, A.; Kul, R. Nitric Oxide Alleviates Copper Toxicity in Germinating Seed and Seedling Growth of Lactuca Sativa L. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2018, 46, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric Oxide Pre-Treatment Advances Seed Germination and Alleviates Copper-Induced Photosynthetic Inhibition in Indian Mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.M.D.O.; de Souza, G.A.; Cardoso, A.; Dias, D.C.F.D.S.; Borges, E.E.D.L.E. Action of Nitric Oxide in Sesame Seeds (Sesamum Indicum L.) Submitted to Stress by Cadmium. J. Seed Sci. 2016, 38, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, H.; Liu, F.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Induction of Chilling Tolerance in Wheat during Germination by Pre-Soaking Seed with Nitric Oxide and Gibberellin. Plant Growth Regul. 2013, 71, 31–40. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Nikzad, K. The Role of Nitric Oxide in Priming-Induced Low-Temperature Tolerance in Two Genotypes of Tomato. Seed Sci. Res. 2013, 23, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.F.; Oliveira dos Santos, H.; Vaz-Tostes, D.P.; Cavasin, P.Y.; Rocha, D.K.; Tirelli, G.V. Protective Action of Priming Agents on Urochloa Brizantha Seeds under Water Restriction and Salinity Conditions. J. Seed Sci. 2021, 43, e202143010. [Google Scholar] [CrossRef]
- Liu, J.; Xue, T.; Shen, Y. Effect of Nitric Oxide on Seed Germination and Dormancy in Empress Trees. Horttechnology 2019, 29, 271–275. [Google Scholar] [CrossRef]
- Yiğit, İ.; Atici, Ö. Seed Priming with Nitric Oxide Mitigates Exogenous Methylglyoxal Toxicity by Restoring Glyoxalase and Antioxidant Systems in Germinating Maize (Zea Mays L.) Seeds. Cereal Res. Commun. 2021, 50, 811–820. [Google Scholar] [CrossRef]
- Marvasi, M. Potential Use and Perspectives of Nitric Oxide Donors in Agriculture. J. Sci. Food Agric. 2017, 97, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Masilamani, P.; Janaki, P.; Eevera, T.; Sundareswaran, S.; Rajkumar, P. Role of Nitric Oxide in Seed Biology and Seed Production: A Review. J. Appl. Nat. Sci. 2020, 12, 277–287. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Chintagunta, A.D.; Reddy, Y.M.; Rajjou, L.; Garlapati, V.K.; Agarwal, D.K.; Prasad, S.R.; Simal-Gandara, J. Implications of Reactive Oxygen and Nitrogen Species in Seed Physiology for Sustainable Crop Productivity under Changing Climate Conditions. Curr. Plant Biol. 2021, 26, 100197. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Babańczyk, T.; Krasuska, U. Nitric Oxide as Germination Controlling Factor in Seeds of Various Plant Species. Phyton 2012, 52, 219–226. [Google Scholar]
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do Nitric Oxide Donors Mimic Endogenous NO-Related Response in Plants? Planta 2006, 224, 1363–1372. [Google Scholar] [CrossRef]
- Chiesa, J.J.; Baidanoff, F.M.; Golombek, D.A. Don’t Just Say No: Differential Pathways and Pharmacological Responses to Diverse Nitric Oxide Donors. Biochem. Pharm. 2018, 156, 1–9. [Google Scholar] [CrossRef]
- Keisham, M.; Jain, P.; Singh, N.; von Toerne, C.; Bhatla, S.C.; Lindermayr, C. Deciphering the Nitric Oxide, Cyanide and Iron-Mediated Actions of Sodium Nitroprusside in Cotyledons of Salt Stressed Sunflower Seedlings. Nitric Oxide 2019, 88, 10–26. [Google Scholar] [CrossRef]
- Arnold, W.P.; Longnecker, D.E.; Epstein, R.M. Photodegradation of Sodium Nitroprusside Biologic Activity and Cyanide Release. Anesthesiology 1984, 61, 254–260. [Google Scholar] [CrossRef]
- Kępczyński, J. Gas-Priming as a Novel Simple Method of Seed Treatment with Ethylene, Hydrogen Cyanide or Nitric Oxide. Acta Physiol. Plant 2021, 43, 117. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Šírová, J.; Sedlářová, M.; Piterková, J.; Luhová, L.; Petřivalský, M. The Role of Nitric Oxide in the Germination of Plant Seeds and Pollen. Plant Sci. 2011, 181, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA Crosstalk with Ethylene and Nitric Oxide in Seed Dormancy and Germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethke, P.C.; Gubler, F.; Jacobsen, J.V.; Jones, R.L. Dormancy of Arabidopsis Seeds and Barley Grains Can Be Broken by Nitric Oxide. Planta 2004, 219, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Libourel, I.G.L.; Jones, R.L. Nitric Oxide Reduces Seed Dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Sarath, G.; Bethke, P.C.; Jones, R.; Baird, L.M.; Hou, G.; Mitchell, R.B. Nitric Oxide Accelerates Seed Germination in Warm-Season Grasses. Planta 2006, 223, 1154–1164. [Google Scholar] [CrossRef] [Green Version]
- Gniazdowska, A.; Dobrzyńska, U.; Babańczyk, T.; Bogatek, R. Breaking the Apple Embryo Dormancy by Nitric Oxide Involves the Stimulation of Ethylene Production. Planta 2007, 225, 1051–1057. [Google Scholar] [CrossRef]
- Kępczyński, J.; Cembrowska-Lech, D.; Sznigir, P. Interplay between Nitric Oxide, Ethylene, and Gibberellic Acid Regulating the Release of Amaranthus Retroflexus Seed Dormancy. Acta Physiol. Plant 2017, 39, 254. [Google Scholar] [CrossRef] [Green Version]
- Sami, A.; Riaz, M.W.; Zhou, X.; Zhu, Z.; Zhou, K. Alleviating Dormancy in Brassica Oleracea Seeds Using NO and KAR1 with Ethylene Biosynthetic Pathway, ROS and Antioxidant Enzymes Modifications. BMC Plant Biol. 2019, 19, 577. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Song, S. Sodium Nitroprusside, Ferricyanide, Nitrite and Nitrate Decrease the Thermo-Dormancy of Lettuce Seed Germination in a Nitric Oxide-Dependent Manner in Light. South Afr. J. Bot. 2012, 78, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Bethke, P.C.; Libourel, I.G.L.; Reinöhl, V.; Jones, R.L. Sodium Nitroprusside, Cyanide, Nitrite, and Nitrate Break Arabidopsis Seed Dormancy in a Nitric Oxide-Dependent Manner. Planta 2006, 223, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Giba, Z.; Grubisic, D.; Konjevic, R. Sodium Nitroprusside-Stimulated Germination of Common Chick Weed (Stellaria Media L.) Seeds. Arch. Biol. Sci. 1992, 44, 17–18. [Google Scholar]
- Li, W.; Liu, X.; Khan, M.A.; Kamiya, Y.; Yamaguchi, S. Hormonal and Environmental Regulation of Seed Germination in Flixweed (Descurainia Sophia). Plant Growth Regul. 2005, 45, 199–207. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric Oxide Stimulates Seed Germination and Counteracts the Inhibitory Effect of Heavy Metals and Salinity on Root Growth of Lupinus Luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Khan, M.A.; Yamaguchi, S. The Effect of Plant Growth Regulators, Nitric Oxide, Nitrate, Nitrite and Light on the Germination of Dimorphic Seeds of Suaeda Salsa under Saline Conditions. J. Plant Res. 2005, 118, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Piterková, J.; Luhová, L.; Hofman, J.; Turecková, V.; Novák, O.; Petrivalsky, M.; Fellner, M. Nitric Oxide Is Involved in Light-Specific Responses of Tomato during Germination under Normal and Osmotic Stress Conditions. Ann. Bot. 2012, 110, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.-P.; Kamiya, Y.; Nambara, E.; Truong, H.-N. The Arabidopsis Abscisic Acid Catabolic Gene CYP707A2 Plays a Key Role in Nitrate Control of Seed Dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Juste, J.; Leon, J. Enhanced Abscisic Acid-Mediated Responses in Nia1nia2noa1-2 Triple Mutant Impaired in NIA/NR- and AtNOA1-Dependent Nitric Oxide Biosynthesis in Arabidopsis. Plant Physiol. 2010, 152, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Gniazdowska, A.; Krasuska, U.; Bogatek, R. Dormancy Removal in Apple Embryos by Nitric Oxide or Cyanide Involves Modifications in Ethylene Biosynthetic Pathway. Planta 2010, 232, 1397–1407. [Google Scholar] [CrossRef]
- Carrillo-Barral, N.; Matilla, A.J.; Iglesias-Fernández, R.; del Carmen Rodríguez-Gacio, M. Nitrate-Induced Early Transcriptional Changes during Imbibition in Non-after-Ripened Sisymbrium Officinale Seeds. Physiol. Plant 2012, 148, 560–573. [Google Scholar] [CrossRef] [Green Version]
- Andryka-Dudek, P.; Ciacka, K.; Wiśniewska, A.; Bogatek, R.; Gniazdowska, A. Nitric Oxide-Induced Dormancy Removal of Apple Embryos Is Linked to Alterations in Expression of Genes Encoding ABA and JA Biosynthetic or Transduction Pathways and RNA Nitration. Int. J. Mol. Sci. 2019, 20, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like Protein 8 Is a Master Regulator of Nitrate-Promoted Seed Germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Md Isa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Marín-de la Rosa, N.; Vicente Conde, J.; Sousa Correia, C.; Pearce, S.P.; et al. Nitric Oxide Sensing in Plants Is Mediated by Proteolytic Control of Group VII ERF Transcription Factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-Barral, N.; Matilla, A.J.; del Carmen Rodríguez-Gacio, M.; Iglesias-Fernández, R. Nitrate Affects Sensu-Stricto Germination of after-Ripened Sisymbrium Officinale Seeds by Modifying Expression of SoNCED5, SoCYP707A2 and SoGA3ox2 Genes. Plant Sci. 2014, 217–218, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Ye, N.; Liu, R.; Jia, W.; Zhang, J. Nitric Oxide-Induced Rapid Decrease of Abscisic Acid Concentration Is Required in Breaking Seed Dormancy in Arabidopsis. New Phytol. 2009, 183, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.; Alqudah, A.M.; Bailly, M.; Rajjou, L.; Pistrick, S.; Matzig, G.; Börner, A.; Kranner, I. Novel Loci and a Role for Nitric Oxide for Seed Dormancy and Preharvest Sprouting in Barley. Plant Cell Env. 2018, 42, 1318–1327. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.L.; Aoyama, N.; Chung, Y.-Y.; Still, D.W.; Jones, R.L. The Arabidopsis Aleurone Layer Responds to Nitric Oxide, Gibberellin, and Abscisic Acid and Is Sufficient and Necessary for Seed Dormancy. Plant Physiol. 2007, 143, 1173–1188. [Google Scholar] [CrossRef] [Green Version]
- Krasuska, U.; Ciacka, K.; Gniazdowska, A. Nitric Oxide-Polyamines Cross-Talk during Dormancy Release and Germination of Apple Embryos. Nitric Oxide 2017, 68, 38–50. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for Blue Light, Jasmonate and Nitric Oxide in the Regulation of Dormancy and Germination in Wheat Grain (Triticum Aestivum L.). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef]
- Ranjan, R.; Lewak, S. Jasmonic Acid Promotes Germination and Lipase Activity in Non-Stratified Apple Embryos. Physiol. Plant 1992, 86, 335–339. [Google Scholar] [CrossRef]
- Lewak, S. Metabolic Control of Embryonic Dormancy in Apple Seed: Seven Decades of Research. Acta Physiol. Plant 2011, 33, 1–24. [Google Scholar] [CrossRef]
- Bailly, C. The Signalling Role of ROS in the Regulation of Seed Germination and Dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Bailly, C.; Merendino, L. Oxidative Signalling in Seed Germination and Early Seedling Growth: An Emerging Role for ROS Trafficking and Inter-Organelle Communication. Biochem. J. 2021, 478, 1977–1984. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants—Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Krasuska, U.; Gniazdowska, A. Nitric Oxide and Hydrogen Cyanide as Regulating Factors of Enzymatic Antioxidant System in Germinating Apple Embryos. Acta Physiol. Plant 2012, 34, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Kolbert, Z.; Feigl, G. Cross-Talk of Reactive Oxygen Species and Nitric Oxide in Various Processes of Plant Development: Past and Present. In Reactive Oxygen Species in Plants: Boon or Bane-Revisiting the Role of ROS; Singh, V.P., Singh, S., Tripathi, D.K., Prasad, S.M., Chauhan, D.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 261–289. ISBN 9781119324928. [Google Scholar]
- Krasuska, U.; Ciacka, K.; Andryka-Dudek, P.; Bogatek, R.; Gniazdowska, A. “Nitrosative Door” in Seed Dormancy Alleviation and Germination. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Signaling and Communication in Plants; Gupta, K.J., Igamberdiev, A.U., Eds.; Springer: Cham, Switzerland, 2015; Volume 23, pp. 215–237. [Google Scholar]
- Krasuska, U.; Dębska, K.; Otulak, K.; Bogatek, R.; Gniazdowska, A. Switch from Heterotrophy to Autotrophy of Apple Cotyledons Depends on NO Signal. Planta 2015, 242, 1221–1236. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Marsolais, F.; Bykova, N.V.; Igamberdiev, A.U. Nitric Oxide and Reactive Oxygen Species Mediate Metabolic Changes in Barley Seed Embryo during Germination. Front. Plant Sci. 2016, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Jasid, S.; Simontacchi, M.; Puntarulo, S. Exposure to Nitric Oxide Protects against Oxidative Damage but Increases the Labile Iron Pool in Sorghum Embryonic Axes. J. Exp. Bot. 2008, 59, 3953–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarath, G.; Hou, G.; Baird, L.M.; Mitchell, R.B. Reactive Oxygen Species, ABA and Nitric Oxide Interactions on the Germination of Warm-Season C4-Grasses. Planta 2007, 226, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 Mediates the Regulation of ABA Catabolism and GA Biosynthesis in Arabidopsis Seed Dormancy and Germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Dębska, K.; Krasuska, U.; Budnicka, K.; Bogatek, R.; Gniazdowska, A. Dormancy Removal of Apple Seeds by Cold Stratification Is Associated with Fluctuation in H2O2, NO Production and Protein Carbonylation Level. J. Plant Physiol. 2013, 170, 480–488. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Krasuska, U.; Czajkowska, K.; Bogatek, R. Nitric Oxide, Hydrogen Cyanide and Ethylene Are Required in the Control of Germination and Undisturbed Development of Young Apple Seedlings. Plant Growth Regul. 2010, 61, 75–84. [Google Scholar] [CrossRef]
- Krasuska, U.; Ciacka, K.; Dębska, K.; Bogatek, R.; Gniazdowska, A. Dormancy Alleviation by NO or HCN Leading to Decline of Protein Carbonylation Levels in Apple (Malus Domestica Borkh.) Embryos. J. Plant Physiol. 2014, 171, 1132–1141. [Google Scholar] [CrossRef]
- Krasuska, U.; Ciacka, K.; Bogatek, R.; Gniazdowska, A. Polyamines and Nitric Oxide Link in Regulation of Dormancy Removal and Germination of Apple (Malus Domestica Borkh.) Embryos. J. Plant Growth Regul. 2014, 33, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Shalimu, D.; Sun, J.; Baskin, C.C.; Baskin, J.M.; Sun, L.; Liu, Y. Changes in Oxidative Patterns during Dormancy Break by Warm and Cold Stratification in Seeds of an Edible Fruit Tree. AoB Plants 2016, 8, plw024. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Bykova, N.V.; Igamberdiev, A.U. Cell Signaling Mechanisms and Metabolic Regulation of Germination and Dormancy in Barley Seeds. Crop. J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Guy, P.A.; Sidaner, J.P.; Schroeder, S.; Edney, M.; MacGregor, A.W.; Hill, R.D. Embryo Phytoglobin Gene Expression as a Measure of Germination in Cereals. J. Cereal. Sci. 2002, 36, 147–156. [Google Scholar] [CrossRef]
- Nie, X.; Mira, M.; Igamberdiev, A.U.; Hill, R.D.; Stasolla, C. Anaerobiosis Modulation of Two Phytoglobins in Barley (Hordeum Vulgare L.), and Their Regulation by Gibberellin and Abscisic Acid in Aleurone Cells. Plant Physiol. Biochem. 2022, 182, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zafari, S.; Hebelstrup, K.H.; Igamberdiev, A.U. Transcriptional and Metabolic Changes Associated with Phytoglobin Expression during Germination of Barley Seeds. Int. J. Mol. Sci. 2020, 21, 2796. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Chaki, M.; Airaki, M.; Valderrama, R.; Palma, J.M.; Barroso, J.B.; Corpas, F.J. Function of S-Nitrosoglutathione Reductase (GSNOR) in Plant Development and under Biotic/Abiotic Stress. Plant Signal Behav. 2011, 6, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Ciacka, K.; Krasuska, U.; Otulak-Kozieł, K.; Gniazdowska, A. Dormancy Removal by Cold Stratification Increases Glutathione and S-Nitrosoglutathione Content in Apple Seeds. Plant Physiol. Biochem. 2019, 138, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, I.; Holzmeister, C.; Wirtz, M.; Geerlof, A.; Fröhlich, T.; Römling, G.; Kuruthukulangarakoola, G.T.; Linster, E.; Hell, R.; Arnold, G.J.; et al. ROS-Mediated Inhibition of S-Nitrosoglutathione Reductase Contributes to the Activation of Anti-Oxidative Mechanisms. Front. Plant Sci. 2016, 7, 1669. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.G.; Chen, J.H.; Kong, X.X.; Todd, C.D.; Yang, Y.P.; Hu, X.Y.; Li, D.Z. Carbon Monoxide Enhances the Chilling Tolerance of Recalcitrant Baccaurea Ramiflora Seeds via Nitric Oxide-Mediated Glutathione Homeostasis. Free Radic. Biol. Med. 2012, 53, 710–720. [Google Scholar] [CrossRef]
- Bai, X.; Yang, L.; Tian, M.; Chen, J.; Shi, J.; Yang, Y.; Hu, X. Nitric Oxide Enhances Desiccation Tolerance of Recalcitrant Antiaris Toxicaria Seeds via Protein S-Nitrosylation and Carbonylation. PLoS ONE 2011, 6, e20714. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhu, J.-K.; Lang, Z. Nitric Oxide Suppresses the Inhibitory Effect of Abscisic Acid on Seed Germination by S-Nitrosylation of SnRK2 Proteins. Plant Signal Behav. 2015, 10, e1031939. [Google Scholar] [CrossRef] [Green Version]
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-Nitrosylation Triggers ABI5 Degradation to Promote Seed Germination and Seedling Growth. Nat. Commun. 2015, 6, 8669. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhu, X.-Y.; Deng, L.-B.; Liu, H.-F.; Li, J.; Zhou, X.-R.; Wang, H.-Z.; Hua, W. Nitric Oxide Affects Seed Oil Accumulation and Fatty Acid Composition through Protein S-Nitrosation. J. Exp. Bot. 2021, 72, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Valderrama, R.; Fernández-Ocaña, A.M.; Carreras, A.; López-Jaramillo, J.; Luque, F.; Palma, J.M.; Pedrajas, J.R.; Begara-Morales, J.C.; Sánchez-Calvo, B.; et al. Protein Targets of Tyrosine Nitration in Sunflower (Helianthus Annuus L.) Hypocotyls. J. Exp. Bot. 2009, 60, 4221–4234. [Google Scholar] [CrossRef]
- Krasuska, U.; Ciacka, K.; Orzechowski, S.; Fettke, J.; Bogatek, R.; Gniazdowska, A. Modification of the Endogenous NO Level Influences Apple Embryos Dormancy by Alterations of Nitrated and Biotinylated Protein Patterns. Planta 2016, 244, 877–891. [Google Scholar] [CrossRef]
- Castillo, M.C.; Lozano-Juste, J.; González-Guzmán, M.; Rodriguez, L.; Rodriguez, P.L.; León, J. Inactivation of PYR/PYL/RCAR ABA Receptors by Tyrosine Nitration May Enable Rapid Inhibition of ABA Signaling by Nitric Oxide in Plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef]
- Vollár, M.; Feigl, G.; Oláh, D.; Horváth, A.; Molnár, Á.; Kúsz, N.; Ördög, A.; Csupor, D.; Kolbert, Z. Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica Napus L. Plants 2020, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Padilla, M.N.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitric Oxide Release from Nitro-Fatty Acids in Arabidopsis Roots. Plant Signal Behav. 2016, 11, e1154255. [Google Scholar] [CrossRef] [Green Version]
- Izbiańska, K.; Floryszak-Wieczorek, J.; Gajewska, J.; Meller, B.; Kuźnicki, D.; Arasimowicz-Jelonek, M. RNA and MRNA Nitration as a Novel Metabolic Link in Potato Immune Response to Phytophthora Infestans. Front Plant Sci. 2018, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Staszek, P.; Gniazdowska, A. Peroxynitrite Induced Signaling Pathways in Plant Response to Non-Proteinogenic Amino Acids. Planta 2020, 252, 5. [Google Scholar] [CrossRef]
- Ciacka, K.; Tymiński, M.; Gniazdowska, A.; Krasuska, U. Carbonylation of Proteins—An Element of Plant Ageing. Planta 2020, 252, 12. [Google Scholar] [CrossRef]
- He, Y.; Xue, H.; Li, Y.; Wang, X. Nitric Oxide Alleviates Cell Death through Protein S-Nitrosylation and Transcriptional Regulation during the Ageing of Elm Seeds. J. Exp. Bot. 2018, 69, 5141–5155. [Google Scholar] [CrossRef] [Green Version]
- Ciacka, K.; Tyminski, M.; Gniazdowska, A.; Krasuska, U. Nitric Oxide as a Remedy against Oxidative Damages in Apple Seeds Undergoing Accelerated Ageing. Antioxidants 2022, 11, 70. [Google Scholar] [CrossRef]
- Mao, C.; Zhu, Y.; Cheng, H.; Yan, H.; Zhao, L.; Tang, J.; Ma, X.; Mao, P. Nitric Oxide Regulates Seedling Growth and Mitochondrial Responses in Aged Oat Seeds. Int. J. Mol. Sci. 2018, 19, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.L.C.; Borges, E.; Oliveira, A.; Leite, H.G.; Gonçalves, J.F. Influence of Nitric Oxide on the Germination of Seeds of Plathymenia Reticulata Benth with Low Vigor. Sci. For. 2010, 38, 629–636. [Google Scholar]
- Rouhi, H.R.; Moradi, A.; Saman, M.; Shahbodaghlo, A.; Mohammadi, Y. Seed Priming with SNP Improves the Performance of Aged Pumpkin (Cucurbita Pepo L.) Seeds under Drought Stress. Iran. J. Seed Sci. Technol. 2019, 8, 67–81. [Google Scholar]
- Chakraborty, S.; Aher, B.M.; Kalyanrao; Rawat, A.; Sasidharan, N. Effect of Potassium Nitrate on Seed Quality Enhancement in Different Aged Seeds of Bottle Gourd (Lagenaria Siceraria (Molina) Standl). J. Exp. Biol. Agric. Sci. 2017, 5, 656–661. [Google Scholar] [CrossRef]
- Sepehri, A.; Rouhi, H.R. Enhancement of Seed Vigor Performance in Aged Groundnut (Arachis Hypogaea L.) Seeds by Sodium Nitroprusside under Drought Stress. Philipp. Agric. Sci. 2016, 99, 339–347. [Google Scholar]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-Wide Characterization of Seed Aging in Arabidopsis: A Comparison between Artificial and Natural Aging Protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [Green Version]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, H.; Fu, Y.-B. Analysis of Stored MRNA Degradation in Acceleratedly Aged Seeds of Wheat and Canola in Comparison to Arabidopsis. Plants 2020, 9, 1707. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Fu, Y.-B.; Wang, H. Arabidopsis Seed Stored MRNAs Are Degraded Constantly over Aging Time, as Revealed by New Quantification Methods. Front. Plant Sci. 2020, 10, 1764. [Google Scholar] [CrossRef]
- Ciacka, K.; Tyminski, M.; Wal, A.; Gniazdowska, A.; Krasuska, U. Nitric Oxide—An Antidote to Seed Aging Modifies Meta-Tyrosine Content and Expression of Aging-Linked Genes in Apple Embryos. Front. Plant Sci. 2022, 13, 2677. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fisher, A.L. Roles of the Tyrosine Isomers Meta-Tyrosine and Ortho-Tyrosine in Oxidative Stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyminski, M.; Ciacka, K.; Staszek, P.; Gniazdowska, A.; Krasuska, U. Toxicity of Meta-Tyrosine. Plants 2021, 10, 2800. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, M.E.; Lemaire, S.D.; Crespo, J.L. Reactive Oxygen Species and Autophagy in Plants and Algae. Plant Physiol. 2012, 160, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Sheen, J. The Role of Target of Rapamycin Signaling Networks in Plant Growth and Metabolism. Plant Physiol. 2014, 164, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bassham, D.C. TOR Is a Negative Regulator of Autophagy in Arabidopsis thaliana. PLoS ONE 2010, 5, e11883. [Google Scholar] [CrossRef]
- Kravchenko, A.; Citerne, S.; Jéhanno, I.; Bersimbaev, R.I.; Veit, B.; Meyer, C.; Leprince, A.-S. Mutations in the Arabidopsis Lst8 and Raptor Genes Encoding Partners of the TOR Complex, or Inhibition of TOR Activity Decrease Abscisic Acid (ABA) Synthesis. Biochem. Biophys. Res. Commun. 2015, 467, 992–997. [Google Scholar] [CrossRef]





| NO Donor | Released Molecules | Conditions of NO Release | References |
|---|---|---|---|
| Sodium nitroprusside (SNP, sodium nitroferricyanide(III) dihydrate) | NO+, CN−, a mixture of ferrocyanide and ferricyanide products | Photolysis after irradiation with UV-Vis light (except red light). Reaction with reducing agents (e.g., thiols, haemoproteins, and ascorbate) in darkness. | [23,24,25] |
| RSNO compounds, e.g., S-nitroso-N-acetyl-D-penicillamine (SNAP) S-nitrosoglutathione (GSNO) | •NO, NO+, NO−, disulphide | Disruption by heat; UV light; and some metal ions, superoxides, and seleno compounds. Metal ions (Cu+, Fe2+, Hg2+, and Ag+) serve as important catalysts for the decomposition of the compounds. | [26,27] |
| 3-Morpholinosydnonimine (SIN-1) | •NO, O2•−, ONOO−, SIN-1C | Decomposition occurs under alkaline pH and is facilitated by oxygen and Vis light irradiation. In vivo, oxidizing agents stimulate •NO production from SIN-1 at low oxygen concentrations. Under such conditions, SIN-1 is likely to behave more like an •NO donor than an ONOO− donor. | [23,26,28] |
| Diazeniumdiolates (NONOates), e.g., Diethylenetriamine NONOate (DETA-NONOate, alternative name NOC-18), | •NO, NO+, NO−, NO2−, nucleophile residue | Decomposition is spontaneous and pH- and temperature-dependent. Dissociation to NO is acid-catalysed, and the rate decreases as the pH increases. | [29,30] |
| Angeli’ salt (sodium α-oxyhyponitrite, belongs to the NONOate compounds) | NO−, •OH, HNO | Spontaneous dissociation in a pH-dependent manner. | [26] |
| Acidified nitrite | 2NO2− + 2H+⇌ N2O3 + H2O N2O3⇌ NO2 + NO | The reaction requires a high NO2− concentration and low pH or the presence of the reductants. e.g., ascorbate. Under less acidic pH, the reduction of NO2− to NO is catalysed by metalloproteins (e.g., haemoglobin). | [31,32] |
| Roussin’s black salt ((KFe4S3(NO)7), the tetra-iron–sulfur-nitrosyl cluster) | NO * and ferric precipitates | Photolysis at λ increases from 313 to 546 nm. | [33] |
| Gene | Changes in Gene Expression | Plant Material | General Description of the Material and Results | References | |
|---|---|---|---|---|---|
| Biosynthesis | NCED6 | ↑/↓ | Hedge mustard seeds | Upregulated at 3 h and downregulated at 22 and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | [83] |
| NCED9 | Upregulated at 12 h and downregulated at 22 and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | ||||
| NCED5 | ↑ | Hedge mustard seeds | Among tested NCED, NCED5 expression the highest during imbibition of 7-month after-ripened seeds and the most affected by 20 mM KNO3. | [88] | |
| NCED6 | Stimulated by 20 mM KNO3 only between 22 and 26 h of imbibition of 7-months after-ripened seeds. | ||||
| NCED9 | |||||
| NCED3 | ↓ | Apple seeds | Downregulated in axes of embryos after 3 h of fumigation with vapours of acidified 20 mM NaNO2. | [84] | |
| NCED9 | |||||
| Degradation | CYP707A2 | ↑ | Arabidopsis seeds | Increased between 3 and 48 h of imbibition after 200 µM SNP application. | [89] |
| CYP707A1 | ↓ | Arabidopsis siliques | Downregulated at 15, 19, and 22 d after flowering and was higher in siliques from a mother plant treated with 10 mM NO3− compared to the plant treated with 3 mM NO3−. | [80] | |
| CYP707A2 | ↑ | Increased at 19 and 22 d after flowering and was higher in siliques from a mother plant treated with 10 mM NO3− compared to the plant treated with 3 mM NO3−. | |||
| CYP707A2 | ↑ | Hedge mustard seeds | Upregulated between 3 and 22 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | [83] | |
| CYP707A2 | ↑ | Hedge mustard seeds | Increased between 3 and 26 h of imbibition of 7-months after-ripened seeds in 20 mM KNO3. | [88] | |
| CYP707A1 | = | Apple seeds | Observed in axes of embryos after 3 h of fumigation with vapours of acidified 20 mM NaNO2. | [84] | |
| CYP707A2 | ↓ | ||||
| Signalling | RCAR3 | ↑ | Apple seeds | Observed in axes of embryos after 3 h of fumigation with vapours of acidified 20 mM NaNO2. | [84] |
| ABF | ↓ | ||||
| ABI5 | ↑/↓ | Hedge mustard seeds | Increased at 12 h and decreased at 3 and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | [83] | |
| ABI5 | ↑ | Hedge mustard seeds | Stimulated by 20 mM KNO3 between 6 and 22 h of imbibition of 7-months after-ripened seeds. | [88] |
| Gene | Changes in Gene Expression | Plant Material | General Description of the Material and Results | References | |
|---|---|---|---|---|---|
| Biosynthesis | GA3ox2 | ↓ | Hedge mustard seeds | Downregulated at 3, 6, 22, and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | [80] |
| GA20ox2 | ↑↓ | Upregulated only at 3 h and downregulated after 6, 12, and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | |||
| GA2ox6 | ↓ | Downregulated after 22 and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | |||
| GA3ox2 | ↑ | Hedge mustard seeds | Stimulated by 20 mM KNO3, especially at 22 and 26 h of imbibition of 7-months after-ripened seeds. | [81] | |
| GA20ox2 | ↑ | Upregulated between 6 and 26 h of imbibition of 7-month after-ripened seeds treated with 20 mM KNO3. | |||
| GA2ox6 | ↑ | ||||
| GA3ox1 | ↓ | Arabidopsis seeds | CN− alleviates the dormancy of Arabidopsis seeds. Seed treatment with 200 µM cPTIO reduced gene expression compared with single CN− application. | [85] | |
| GA3ox2 | |||||
| Signalling | RGL2 | ↓ | Hedge mustard seeds | Downregulated after 22 and 26 h of imbibition of non-after-ripened seeds in 20 mM KNO3. | [80] |
| RGL2 | ↓ | Hedge mustard seeds | In 7-months after-ripened seeds, expression was negatively affected by 20 mM KNO3 at 6 and 22 h of imbibition. | [81] |
| Gene | Changes in Gene Expression | Plant Material | General Description of the Material and Results | References | |
|---|---|---|---|---|---|
| Biosynthesis | ACS7 | ↑ | Hedge mustard seeds | After 20 mM KNO3 treatment, the ACS7 transcript was expressed only at the beginning of imbibition (3 h) in seeds that were not after-ripened, and after-ripening eliminated this expression. | [86] |
| ACO2 | ↑/↓ | After 20 mM KNO3 treatment, the transcript level was very high in non-after-ripened seeds at 3 h of imbibition and strongly diminished up to 12 h, increasing afterwards; after-ripening reduced transcript accumulation during the first 6 h of imbibition. | |||
| ACS1 | ↑ | Wild cabbage seeds | Upregulated between 12 and 48 h of imbibition in seeds treated with 5 mM NO. | [72] | |
| ACS3 | |||||
| ACS7 | |||||
| ACS11 | |||||
| ACS9 | Increased at 12 and 24 h of imbibition of seeds treated with 5 mM NO. | ||||
| ACS4 | ↓ | Decreased between 12 and 48 h of imbibition in seeds treated with 5 mM NO. | |||
| ACS5 | |||||
| ACO1 | ↓/↑ | Downregulated after 12 h and upregulated after 24 and 36 h of imbibition in seeds treated with 5 mM NO. | |||
| Signalling | ETR1 | ↑ | Wild cabbage seeds | Upregulated between 12 and 48 h of imbibition in seeds treated with 5 mM NO. | [72] |
| ETR2 |
| Gene | Changes in Gene Expression | Plant Material | General Description of the Material and Results | References | |
|---|---|---|---|---|---|
| Biosynthesis | AOS1 | ↑ | Apple seeds | Observed in axes of embryos after 3 h of fumigation with vapours of acidified 20 mM NaNO2. | [82] |
| Derivative formation | JMT | ↑ | |||
| JAR1 | ↓ | ||||
| Signalling | JAZ3 | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciacka, K.; Staszek, P.; Sobczynska, K.; Krasuska, U.; Gniazdowska, A. Nitric Oxide in Seed Biology. Int. J. Mol. Sci. 2022, 23, 14951. https://doi.org/10.3390/ijms232314951
Ciacka K, Staszek P, Sobczynska K, Krasuska U, Gniazdowska A. Nitric Oxide in Seed Biology. International Journal of Molecular Sciences. 2022; 23(23):14951. https://doi.org/10.3390/ijms232314951
Chicago/Turabian StyleCiacka, Katarzyna, Pawel Staszek, Katarzyna Sobczynska, Urszula Krasuska, and Agnieszka Gniazdowska. 2022. "Nitric Oxide in Seed Biology" International Journal of Molecular Sciences 23, no. 23: 14951. https://doi.org/10.3390/ijms232314951