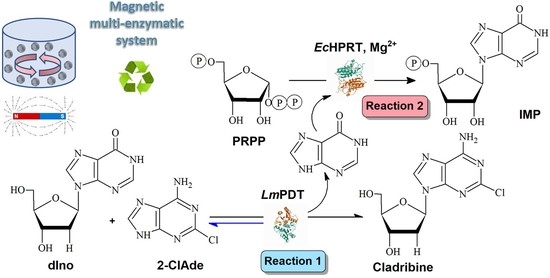
Magnetic Multi-Enzymatic System for Cladribine Manufacturing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzyme Immobilization
2.2. Biochemical Characterization of Immobilized Derivatives
2.3. Biochemical Characterization of the MLmPDT3/MEcHPRT3 System
2.4. Thermal Stability of the Magnetic MLmPDT3/MEcHPRT3 System
2.5. Application of the MLmPDT3/MEcHPRT3 System in Manufacturing Cladribine and IMP
3. Materials and Methods
3.1. Materials
3.2. Gene Expression and Protein Purification
3.3. Enzyme Immobilization
3.4. N-Deoxyribosyltransferase Assay
3.5. Phosphoribosyltransferase Activity Assay
3.6. Biochemical Characterization of Immobilized Derivatives
3.7. MLmPDT3/MEcHPRT3 Standard Assay
3.8. Biochemical Characterization of the MLmPDT3/MEcHPRT3 System
3.9. Thermal Inactivation of the MLmPDT3/MEcHPRT3 System
3.10. Application of the MLmPDT3/MEcHPRT3 System in the Manufacturing of Cladribine and IMP
3.11. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fernández-Lucas, J.; Camarasa, M.J. (Eds.) Enzymatic and Chemical Synthesis of Nucleic acid Derivatives; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Simić, S.; Zukić, E.; Schmermund, L.; Faber, K.; Winkler, C.K.; Kroutil, W. Shortening synthetic routes to small molecule active pharmaceutical ingredients employing biocatalytic methods. Chem. Rev. 2021, 122, 1052–1126. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Rivero, C.W.; Zinni, M.A.; Britos, C.N.; Trelles, J.A. New developments in nucleoside analogues biosynthesis: A review. J. Mol. Catal. B Enzym. 2016, 133, 218–233. [Google Scholar] [CrossRef]
- Del Arco, J.; Acosta, J.; Fernández-Lucas, J. New trends in the biocatalytic production of nucleosidic active pharmaceutical ingredients using 2′-deoxyribosyltransferases. Biotechnol. Adv. 2021, 51, 107701. [Google Scholar] [CrossRef] [PubMed]
- Mikhailopulo, I.A.; Miroshnikov, A.I. New trends in nucleoside biotechnology. Acta Nat. 2010, 2, 36–58. [Google Scholar] [CrossRef] [Green Version]
- Lewkowicz, E.S.; Iribarren, A.M. Whole cell biocatalysts for the preparation of nucleosides and their derivatives. Curr. Pharm. Des. 2017, 23, 6851–6878. [Google Scholar] [CrossRef]
- Rinaldi, F.; Fernández-Lucas, J.; de la Fuente, D.; Zheng, C.; Bavaro, T.; Peters, B.; Massolini, G.; Annunziata, F.; Conti, P.; De la Mata, I.; et al. Immobilized enzyme reactors based on nucleoside phosphorylases and 2’-deoxyribosyltransferase for the in-flow synthesis of pharmaceutically relevant nucleoside analogues. Bioresour. Technol. 2020, 307, 123258. [Google Scholar] [CrossRef]
- Kamel, S.; Weiß, M.; Klare, H.F.; Mikhailopulo, I.A.; Neubauer, P.; Wagner, A. Chemo-enzymatic synthesis of α-D-pentofuranose-1-phosphates using thermostable pyrimidine nucleoside phosphorylases. Mol. Catal. 2018, 458, 52–59. [Google Scholar] [CrossRef]
- Fresco-Taboada, A.; De La Mata, I.; Arroyo, M.; Fernández-Lucas, J. New insights on nucleoside 2′-deoxyribosyltransferases: A versatile biocatalyst for one-pot one-step synthesis of nucleoside analogs. Appl. Microbiol. Biotechnol. 2013, 97, 3773–3785. [Google Scholar] [CrossRef]
- Del Arco, J.; Fernández-Lucas, J. Purine and pyrimidine phosphoribosyltransferases: A versatile tool for enzymatic synthesis of nucleoside-5′-monophosphates. Curr. Pharm. Des. 2017, 23, 6898–6912. [Google Scholar] [CrossRef]
- Del Arco, J.; Cejudo-Sanches, J.; Esteban, I.; Clemente-Suárez, V.J.; Hormigo, D.; Perona, A.; Fernández-Lucas, J. Enzymatic production of dietary nucleotides from low-soluble purine bases by an efficient, thermostable and alkali-tolerant biocatalyst. Food Chem. 2017, 237, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, J.; Pérez, E.; Naitow, H.; Kunishima, N.; Fernández-Lucas, J. Structural and functional characterization of thermostable biocatalysts for the synthesis of 6-aminopurine nucleoside-5′-monophospate analogues. Bioresour. Technol. 2019, 276, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Mikhailopulo, I.A.; Miroshnikov, A.I. Biologically important nucleosides: Modern trends in Biotechnology and application. Mendeleev Commun. 2011, 21, 57–69. [Google Scholar] [CrossRef]
- Frisch, J.; Maršić, T.; Loderer, C. A novel one-pot enzyme cascade for the biosynthesis of cladribine triphosphate. Biomolecules 2021, 11, 346. [Google Scholar] [CrossRef]
- Ding, Y.; Ou, L.; Ding, Q. Enzymatic synthesis of nucleoside triphosphates and deoxynucleoside triphosphates by surface-displayed kinases. Appl. Biochem. Biotechnol. 2020, 190, 1271–1288. [Google Scholar] [CrossRef]
- Spurgeon, S.; Yu, M.; Phillips, J.D.; Epner, E.M. Cladribine: Not just another purine analogue? Expert Opin. Investig. Drugs 2002, 18, 1169–1181. [Google Scholar] [CrossRef]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Medicinal chemistry of multiple sclerosis: Focus on cladribine. Mini Rev. Med. Chem. 2020, 20, 269–285. [Google Scholar] [CrossRef]
- Britos, C.N.; Lapponi, M.J.; Cappa, V.A.; Rivero, C.W.; Trelles, J.A. Biotransformation of halogenated nucleosides by immobilized Lactobacillus animalis 2′-N-deoxyribosyltransferase. J. Fluor. Chem. 2016, 186, 91–96. [Google Scholar] [CrossRef]
- Drenichev, M.S.; Alexeev, C.S.; Kurochkin, N.N.; Mikhailov, S.N. Use of nucleoside phosphorylases for the preparation of purine and pyrimidine 2′-deoxynucleosides. Adv. Synth. Catal. 2018, 360, 305–312. [Google Scholar] [CrossRef]
- Liu, G.; Cheng, T.; Chu, J.; Li, S.; He, B. Efficient synthesis of purine nucleoside analogs by a new trimeric purine nucleoside phosphorylase from Aneurinibacillus migulanus AM007. Molecules 2020, 25, 100. [Google Scholar] [CrossRef]
- Rabuffetti, M.; Bavaro, T.; Semproli, R.; Cattaneo, G.; Massone, M.; Morelli, C.F.; Speranza, G.; Ubiali, D. Synthesis of ribavirin, tecadenoson, and cladribine by enzymatic transglycosylation. Catalysts 2019, 9, 355. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Szeker, K.; Jiao, L.Y.; Oestreich, M.; Mikhailopulo, I.A.; Neubauer, P. Synthesis of 2,6-dihalogenated purine nucleosides by thermostable nucleoside phosphorylases. Adv. Synth. Catal. 2015, 357, 1237–1244. [Google Scholar] [CrossRef]
- Acosta, J.; Del Arco, J.; Martinez-Pascual, S.; Clemente-Suárez, V.J.; Fernández-Lucas, J. One-pot multi-enzymatic production of purine derivatives with application in pharmaceutical and food industry. Catalysts 2018, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Pérez, E.; Sánchez-Murcia, P.A.; Jordaan, J.; Blanco, M.D.; Mancheño, J.M.; Gago, F.; Fernández-Lucas, J. Enzymatic synthesis of therapeutic nucleosides using a highly versatile purine nucleoside 2′-deoxyribosyltransferase from Trypanosoma brucei. ChemCatChem 2018, 10, 4406–4416. [Google Scholar] [CrossRef]
- Rivero, C.W.; García, N.S.; Fernández-Lucas, J.; Betancor, L.; Romanelli, G.P.; Trelles, J. Green production of cladribine by using immobilized 2′-deoxyribosyltransferase from Lactobacillus delbrueckii stabilized through a double covalent/entrapment technology. Biomolecules 2021, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernández-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [Green Version]
- Fresco-Taboada, A.; Fernández-Lucas, J.; Acebal, C.; Arroyo, M.; Ramón, F.; De la Mata, I.; Mancheño, J.M. 2′-Deoxyribosyltransferase from Bacillus psychrosaccharolyticus: A mesophilic-like biocatalyst for the synthesis of modified nucleosides from a psychrotolerant bacterium. Catalysts 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Borlido, L.; Azevedo, A.M.; Roque, A.C.A.; Aires-Barros, M.R. Magnetic separations in biotechnology. Biotechnol. Adv. 2013, 31, 1374–1385. [Google Scholar] [CrossRef]
- Del Arco, J.; Alcántara, A.R.; Fernández-Lafuente, R.; Fernández-Lucas, J. Magnetic micro-macro biocatalysts applied to industrial bioprocesses. Bioresour. Technol. 2021, 322, 124547. [Google Scholar] [CrossRef]
- Crespo, N.; Sánchez-Murcia, P.A.; Gago, F.; Cejudo-Sanches, J.; Galmes, M.A.; Fernández-Lucas, J.; Mancheño, J.M. 2′-Deoxyribosyltransferase from Leishmania mexicana, an efficient biocatalyst for one-pot, one-step synthesis of nucleosides from poorly soluble purine bases. Appl. Microbiol. Biotechnol. 2017, 101, 7187–7200. [Google Scholar] [CrossRef]
- Guddat, L.W.; Vos, S.; Martin, J.L.; Keough, D.T.; de Jersey, J. Crystal structures of free, IMP-, and GMP-bound Escherichia coli hypoxanthine phosphoribosyltransferase. Prot. Sci. 2002, 11, 1626–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keough, D.T.; Hocková, D.; Rejman, D.; Špaček, P.; Vrbková, S.; Krečmerová, M.; Eng, W.S.; Jans, H.; West, N.P.; Naesens, L.M.J.; et al. Inhibition of the Escherichia coli 6-oxopurine phosphoribosyltransferases by nucleoside phosphonates: Potential for new antibacterial agents. J. Med. Chem. 2013, 56, 6967–6984. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Nguyen, K.; Spitale, R.C.; Fernández-Lucas, J. Taylor-made production of pyrimidine nucleoside-5′-monophosphate analogues by highly stabilized mutant uracil phosphoribosyltransferase from Toxoplasma gondii. Bioresour. Technol. 2021, 339, 125649. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, J.; Jordaan, J.; Moral-Dardé, V.; Fernández-Lucas, J. Sustainable production of nucleoside analogues by a high-efficient purine 2′-deoxyribosyltransferase immobilized onto Ni2+ chelate magnetic microparticles. Bioresour. Technol. 2019, 289, 121772. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Fresco-Taboada, A.; Acebal, C.; de la Mata, I.; Arroyo, M. Enzymatic synthesis of nucleoside analogues using immobilized 2′-deoxyribosyltransferase from Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2011, 91, 317–327. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Fresco-Taboada, A.; de la Mata, I.; Arroyo, M. One-step enzymatic synthesis of nucleosides from low water-soluble purine bases in non-conventional media. Bioresour. Technol. 2012, 115, 63–69. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Harris, R.; Mata-Casar, I.; Heras, A.; de la Mata, I.; Arroyo, M. Magnetic chitosan beads for covalent immobilization of nucleoside 2′-deoxyribosyltransferase: Application in nucleoside analogues synthesis. J. Ind. Microbiol. Biotechnol. 2013, 40, 955–966. [Google Scholar] [CrossRef]
- Fresco-Taboada, A.; Serra, I.; Fernández-Lucas, J.; Acebal, C.; Arroyo, M.; Terreni, M.; De la Mata, I. Nucleoside 2’-deoxyribosyltransferase from psychrophilic bacterium Bacillus psychrosaccharolyticus—Preparation of an immobilized biocatalyst for the enzymatic synthesis of therapeutic nucleosides. Molecules 2014, 19, 11231–11249. [Google Scholar] [CrossRef]
- Fresco-Taboada, A.; Serra, I.; Arroyo, M.; Fernández-Lucas, J.; de la Mata, I.; Terreni, M. Development of an immobilized biocatalyst based on Bacillus psychrosaccharolyticus NDT for the preparative synthesis of trifluridine and decytabine. Catal. Today 2016, 259, 197–204. [Google Scholar] [CrossRef]
- Del Arco, J.; Martínez-Pascual, S.; Clemente-Suárez, V.J.; Corral, O.J.; Jordaan, J.; Hormigo, D.; Perona, A.; Fernández-Lucas, J. One-pot, one-step production of dietary nucleotides by magnetic biocatalysts. Catalysts 2018, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. Chem. Cat. Chem. 2015, 7, 2413–2432. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and umami taste: Natural products, their chemosensory targets, and beyond. Angew. Chem. Int. Ed. 2011, 50, 2220–2242. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Pérez, E.; Sánchez-Murcia, P.A.; Fillat, C.; Fernández-Lucas, J. Molecular basis of NDT-mediated activation of nucleoside-based prodrugs and application in suicide gene therapy. Biomolecules 2021, 11, 120. [Google Scholar] [CrossRef]






| Reaction 1 | Reaction 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Substrates | Product | Conversion (%) | Substrates | Product | Conversion (%) | ||||
| dIno [mM] | 2-ClAde [mM] | Cladribine [mM] |
Hyp [mM] |
PRPP [mM] |
IMP [mM] | ||||
| MLmPDT3 a | 1 | 1 | 0.40 ± 0.03 | 40 | - | - | - | - | |
| MLmPDT3/MEcHPRT3 b | 1 | 1 | 0.52 ± 0.04 | 52 | 0.52 | 1 | 0.39 ± 0.01 | 75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, G.; Saiz, L.P.; Bilal, M.; Eltoukhy, L.; Loderer, C.; Fernández-Lucas, J. Magnetic Multi-Enzymatic System for Cladribine Manufacturing. Int. J. Mol. Sci. 2022, 23, 13634. https://doi.org/10.3390/ijms232113634
Cruz G, Saiz LP, Bilal M, Eltoukhy L, Loderer C, Fernández-Lucas J. Magnetic Multi-Enzymatic System for Cladribine Manufacturing. International Journal of Molecular Sciences. 2022; 23(21):13634. https://doi.org/10.3390/ijms232113634
Chicago/Turabian StyleCruz, Guillermo, Laura Pilar Saiz, Muhammad Bilal, Lobna Eltoukhy, Christoph Loderer, and Jesús Fernández-Lucas. 2022. "Magnetic Multi-Enzymatic System for Cladribine Manufacturing" International Journal of Molecular Sciences 23, no. 21: 13634. https://doi.org/10.3390/ijms232113634