Neuroprotective Effects of Resveratrol by Modifying Cholesterol Metabolism and Aβ Processing in SAMP8 Mice
Abstract
:1. Introduction
2. Results
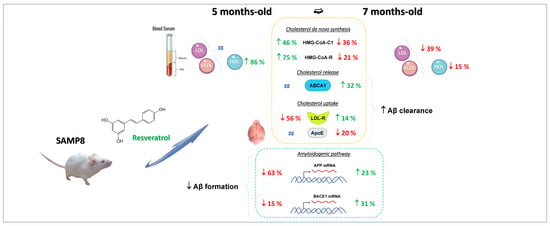
2.1. Levels of Free Cholesterol in the Brain of SAMP8 Mice
2.2. Cholesterol Uptake and Release in the Brain of SAMP8 Mice
2.3. Levels of Cholesterol-Carrier ApoE in the Brain of SAMP8 Mice
2.4. Endogenous Synthesis of Cholesterol in the Brain of SAMP8 Mice
2.5. Quantification of BACE-1 and APP Levels in the Brain from SAMP8 Mice
2.6. Levels of Free Cholesterol and Lipoproteins in Blood Serum from SAMP8 Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Resveratrol Diet
4.2. Blood Serum Collection
4.3. Brain Extraction and Plasma Membrane Isolation
4.4. Total RNA Extraction and cDNA Preparation
4.5. Gene Expression Analysis by Real-Time PCR
4.6. Immunodetection by Western Blotting Assay
4.7. Quantification of Free Cholesterol and Lipoproteins
4.8. Statistical and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borisova, T.; Krisanova, N.; Sivko, R.; Borysov, A. Cholesterol depletion attenuates tonic release but increases the ambient level of glutamate in rat brain synaptosomes. Neurochem. Int. 2010, 56, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Gidding, S.S.; Allen, N.B. Cholesterol and atherosclerotic cardiovascular disease: A lifelong problem. J. Am. Heart Assoc. 2019, 8, e012924. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The role of cholesterol in cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenas, F.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Intracellular cholesterol trafficking and impact in neurodegeneration. Front. Mol. Neurosci. 2017, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef]
- Vance, J.E. Dysregulation of cholesterol balance in the brain: Contribution to neurodegenerative diseases. Dis. Model. Mech. 2012, 5, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.M.; Teplow, D.B. Alzheimer’s disease and the amyloid beta-protein. Prog. Mol. Biol. Transl. Sci. 2012, 107, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, K.; Wallin, A.; Blennow, K. Effect of statins on beta-amyloid metabolism in humans: Potential importance for the development of senile plaques in alzheimer’s disease. Acta Neurol. Scand. Suppl. 2006, 185, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, Y.; Yamazaki, N.; Nagaoka, A.; Matsuo, T.; Nagawa, Y.; Takeda, T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 1986, 38, 399–406. [Google Scholar] [CrossRef]
- Akiguchi, I.; Pallas, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H.; et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Liu, J.; Shi, J.S. SAMP8 mice as a model of age-related cognition decline with underlying mechanisms in alzheimer’s disease. J. Alzheimers Dis. 2020, 75, 385–395. [Google Scholar] [CrossRef]
- Cheng, X.R.; Zhou, W.X.; Zhang, Y.X. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an alzheimer’s disease animal model. Ageing Res. Rev. 2014, 13, 13–37. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, T.; Ma, Y.; Huang, S.; Lei, L.; Wen, A.; Ding, Y. Resveratrol: Multi-targets mechanism on neurodegenerative diseases based on network pharmacology. Front. Pharmacol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Komorowska, J.; Watroba, M.; Szukiewicz, D. Review of beneficial effects of resveratrol in neurodegenerative diseases such as alzheimer’s disease. Adv. Med. Sci. 2020, 65, 415–423. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Li, J.; Zhang, C. Resveratrol ameliorates spatial learning memory impairment induced by Abeta1-42 in rats. Neuroscience 2017, 344, 39–47. [Google Scholar] [CrossRef]
- Sarroca, S.; Gatius, A.; Rodriguez-Farre, E.; Vilchez, D.; Pallas, M.; Grinan-Ferre, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Chen, T.G.; Tai, Y.T.; Chen, T.L.; Chiu, W.T.; Chen, R.M. Resveratrol attenuates oxidized LDL-evoked Lox-1 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cells. J. Cereb. Blood Flow Metab. 2011, 31, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, J.; Zhu, W.; Yin, X.; Yang, B.; Wei, Y.; Guo, X. Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 2018, 19, 3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Albasanz, J.L.; Pallas, M.; Martin, M. Adenosine metabolism in the cerebral cortex from several mice models during aging. Int. J. Mol. Sci. 2020, 21, 7300. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Albasanz, J.L.; Palomera-Avalos, V.; Pallas, M.; Martin, M. Resveratrol modulates and reverses the age-related effect on adenosine-mediated signalling in SAMP8 mice. Mol. Neurobiol. 2019, 56, 2881–2895. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Albasanz, J.L.; Pallas, M.; Martin, M. Resveratrol differently modulates group I metabotropic glutamate receptors depending on age in SAMP8 mice. ACS Chem. Neurosci. 2020, 11, 1770–1780. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Izquierdo-Ramirez, P.J.; Palomera-Avalos, V.; Pallas, M.; Albasanz, J.L.; Martin, M. High-fat and resveratrol supplemented diets modulate adenosine receptors in the cerebral cortex of C57BL/6J and SAMP8 mice. Nutrients 2021, 13, 3040. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Albasanz, J.L.; Grinan-Ferre, C.; Pallas, M.; Martin, M. Adenosine and metabotropic glutamate receptors are present in blood serum and exosomes from SAMP8 mice: Modulation by aging and resveratrol. Cells 2020, 9, 1628. [Google Scholar] [CrossRef]
- Petrov, A.M.; Kasimov, M.R.; Zefirov, A.L. Brain cholesterol metabolism and its defects: Linkage to neurodegenerative diseases and synaptic dysfunction. Acta Nat. 2016, 8, 58–73. [Google Scholar] [CrossRef] [Green Version]
- Grosgen, S.; Grimm, M.O.; Friess, P.; Hartmann, T. Role of amyloid beta in lipid homeostasis. Biochim. Biophys. Acta 2010, 1801, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Casadesus, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegri, C.; Sanfeliu, C.; Camins, A.; Pallas, M.; et al. Dietary resveratrol prevents alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flurkey, K.; Currer, J.; Harrison, D. The mouse in aging research. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Ed.; American College Laboratory Animal Medicine (Elsevier): Burlington, MA, USA, 2007; pp. 637–672. [Google Scholar]
- Guixa-Gonzalez, R.; Albasanz, J.L.; Rodriguez-Espigares, I.; Pastor, M.; Sanz, F.; Marti-Solano, M.; Manna, M.; Martinez-Seara, H.; Hildebrand, P.W.; Martin, M.; et al. Membrane cholesterol access into a G-protein-coupled receptor. Nat. Commun. 2017, 8, 14505. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Weaver, D.F. Axonal plasma membrane-mediated toxicity of cholesterol in alzheimer’s disease: A microsecond molecular dynamics study. Biophys Chem. 2021, 281, 106718. [Google Scholar] [CrossRef]
- Perez-Canamas, A.; Sarroca, S.; Melero-Jerez, C.; Porquet, D.; Sansa, J.; Knafo, S.; Esteban, J.A.; Sanfeliu, C.; Ledesma, M.D. A diet enriched with plant sterols prevents the memory impairment induced by cholesterol loss in senescence-accelerated mice. Neurobiol. Aging 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Petrov, A.M.; Kasimov, M.R.; Zefirov, A.L. Cholesterol in the pathogenesis of alzheimer’s, parkinson’s diseases and autism: Link to synaptic dysfunction. Acta Nat. 2017, 9, 26–37. [Google Scholar] [CrossRef]
- Chang, T.Y.; Yamauchi, Y.; Hasan, M.T.; Chang, C. Cellular cholesterol homeostasis and alzheimer’s disease. J. Lipid Res. 2017, 58, 2239–2254. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.O.W.; Michaelson, D.M.; Hartmann, T. Omega-3 fatty acids, lipids, and apoE lipidation in alzheimer’s disease: A rationale for multi-nutrient dementia prevention. J. Lipid Res. 2017, 58, 2083–2101. [Google Scholar] [CrossRef] [Green Version]
- Olmastroni, E.; Molari, G.; De Beni, N.; Colpani, O.; Galimberti, F.; Gazzotti, M.; Zambon, A.; Catapano, A.L.; Casula, M. Statin use and risk of dementia or alzheimer’s disease: A systematic review and meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2021, 29, 804–814. [Google Scholar] [CrossRef]
- Dutta, S.; Rahman, S.; Ahmad, R.; Kumar, T.; Dutta, G.; Banerjee, S.; Abubakar, A.R.; Rowaiye, A.B.; Dhingra, S.; Ravichandiran, V.; et al. An evidence-based review of neuronal cholesterol role in dementia and statins as a pharmacotherapy in reducing risk of dementia. Expert Rev. Neurother. 2021, 21, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Shin, D.W.; Yoo, T.G.; Cho, M.H.; Jang, W.; Lee, J.; Kim, S. Association between statin use and alzheimer’s disease with dose response relationship. Sci. Rep. 2021, 11, 15280. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, E.A.; Kim, Y.S.; Kim, Y.; You, H.S.; Han, Y.E.; Kim, H.S.; Bae, Y.J.; Kim, J.; Kang, H.T. Statin exposure and the risk of dementia in individuals with hypercholesterolaemia. J. Intern Med. 2020, 288, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Bostrom, K.; Jungbjer, B.; Olsson, L. Membrane lipids of adult human brain: Lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 1994, 63, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Callaghan, D.; Jones, A.; Walker, D.G.; Lue, L.F.; Beach, T.G.; Sue, L.I.; Woulfe, J.; Xu, H.; Stanimirovic, D.B.; et al. Cholesterol retention in alzheimer’s brain is responsible for high beta- and gamma-secretase activities and abeta production. Neurobiol. Dis. 2008, 29, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Prasanthi, J.R.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Herrera, F.; Taoro-Gonzalez, L.; Valdes-Baizabal, C.; Diaz, M.; Marin, R. Lipid and lipid raft alteration in aging and neurodegenerative diseases: A window for the development of new biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [Green Version]
- Berrougui, H.; Isabelle, M.; Cloutier, M.; Grenier, G.; Khalil, A. Age-related impairment of HDL-mediated cholesterol efflux. J. Lipid Res. 2007, 48, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Kumar, V.B.; Farr, S.A.; Flood, J.F.; Kamlesh, V.; Franko, M.; Banks, W.A.; Morley, J.E. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides 2000, 21, 1769–1775. [Google Scholar] [CrossRef]
- Morley, J.E.; Kumar, V.B.; Bernardo, A.E.; Farr, S.A.; Uezu, K.; Tumosa, N.; Flood, J.F. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides 2000, 21, 1761–1767. [Google Scholar] [CrossRef]
- Grinan-Ferre, C.; Palomera-Avalos, V.; Puigoriol-Illamola, D.; Camins, A.; Porquet, D.; Pla, V.; Aguado, F.; Pallas, M. Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp. Gerontol. 2016, 80, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.W.; Cheng, X.R.; Cheng, J.P.; Zhou, W.X.; Zhang, Y.X. The activity and mRNA expression of beta-secretase, cathepsin D, and cathepsin B in the brain of senescence-accelerated mouse. J. Alzheimers Dis. 2012, 28, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Rosene, D.L.; Moss, M.B.; Raju, S.; Hyman, B.T.; Irizarry, M.C. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am. J. Pathol. 2004, 164, 719–725. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yasvoina, M.; Shao, P.; Hitt, B.; O’Connor, T.; Logan, S.; Maus, E.; Citron, M.; Berry, R.; et al. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: Implications for alzheimer’s disease pathogenesis. J. Neurosci. 2007, 27, 3639–3649. [Google Scholar] [CrossRef]
- Zohar, O.; Pick, C.G.; Cavallaro, S.; Chapman, J.; Katzav, A.; Milman, A.; Alkon, D.L. Age-dependent differential expression of BACE splice variants in brain regions of tg2576 mice. Neurobiol. Aging 2005, 26, 1167–1175. [Google Scholar] [CrossRef]
- Apelt, J.; Bigl, M.; Wunderlich, P.; Schliebs, R. Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int. J. Dev. Neurosci. 2004, 22, 475–484. [Google Scholar] [CrossRef]
- Wang, H.; Kulas, J.A.; Wang, C.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. USA 2021, 118, e2102191118. [Google Scholar] [CrossRef]
- Grimm, M.O.; Mett, J.; Grimm, H.S.; Hartmann, T. APP function and lipids: A bidirectional link. Front. Mol. Neurosci. 2017, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.O.; Rothhaar, T.L.; Hartmann, T. The role of APP proteolytic processing in lipid metabolism. Exp. Brain Res. 2012, 217, 365–375. [Google Scholar] [CrossRef]
- Barrett, P.J.; Song, Y.; Van Horn, W.D.; Hustedt, E.J.; Schafer, J.M.; Hadziselimovic, A.; Beel, A.J.; Sanders, C.R. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 2012, 336, 1168–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and neuroprotection: Impact and its therapeutic potential in alzheimer’s disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef] [PubMed]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid beta1-42 peptide: Potential relevance to alzheimer’s disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, N.; Liu, X. Resveratrol and amyloid-beta: Mechanistic insights. Nutrients 2017, 9, 1122. [Google Scholar] [CrossRef] [Green Version]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef] [Green Version]
- Castellano, J.M.; Deane, R.; Gottesdiener, A.J.; Verghese, P.B.; Stewart, F.R.; West, T.; Paoletti, A.C.; Kasper, T.R.; DeMattos, R.B.; Zlokovic, B.V.; et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Abeta clearance in a mouse model of beta-amyloidosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15502–15507. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Andhey, P.S.; Ising, C.; Wang, K.; Snipes, L.L.; Boyer, K.; Lawson, S.; Yamada, K.; Qin, W.; Manis, M.; et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron 2021, 109, 2413.e2417–2426.e2417. [Google Scholar] [CrossRef]
- Kim, J.; Castellano, J.M.; Jiang, H.; Basak, J.M.; Parsadanian, M.; Pham, V.; Mason, S.M.; Paul, S.M.; Holtzman, D.M. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular a beta clearance. Neuron 2009, 64, 632–644. [Google Scholar] [CrossRef] [Green Version]
- Basak, J.M.; Verghese, P.B.; Yoon, H.; Kim, J.; Holtzman, D.M. Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of abeta uptake and degradation by astrocytes. J. Biol. Chem. 2012, 287, 13959–13971. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural insights and links to alzheimer disease pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef]
- Li, Y.; Macyczko, J.R.; Liu, C.C.; Bu, G. ApoE4 reduction: An emerging and promising therapeutic strategy for alzheimer’s disease. Neurobiol. Aging 2022, 115, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, C.T.; Laham, M.S.; Thatcher, G.R.J. Remembering your A, B, C’s: Alzheimer’s disease and ABCA1. Acta Pharm. Sin. B 2022, 12, 995–1018. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Hai, O.; Littlefield, M.J.; Carsons, S.; Reiss, A.B. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARgamma and adenosine. Eur. J. Pharmacol. 2013, 698, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Sevov, M.; Elfineh, L.; Cavelier, L.B. Resveratrol regulates the expression of LXR-alpha in human macrophages. Biochem. Biophys. Res. Commun. 2006, 348, 1047–1054. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Albasanz, J.L.; Guixa-Gonzalez, R.; Saleh, N.; Selent, J.; Martin, M. The antioxidant resveratrol acts as a non-selective adenosine receptor agonist. Free Radic. Biol. Med. 2019, 135, 261–273. [Google Scholar] [CrossRef]
- Lv, Z.M.; Ling, M.Y.; Chen, C. Comparative proteomics reveals protective effect of resveratrol on a high-fat diet-induced damage to mice testis. Syst. Biol. Reprod. Med. 2020, 66, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Wang, Y.; Huang, Q.; Shi, J.; Yang, H.; Pan, Z.; Jin, M.; Zhao, H.; Xu, X. Cholesterol-lowering effects and mechanisms in view of bile acid pathway of resveratrol and resveratrol glucuronides. J. Food Sci. 2016, 81, H2841–H2848. [Google Scholar] [CrossRef]
- Mohamed, H.E.; El-Swefy, S.E.; Hasan, R.A.; Hasan, A.A. Neuroprotective effect of resveratrol in diabetic cerebral ischemic-reperfused rats through regulation of inflammatory and apoptotic events. Diabetol. Metab. Syndr. 2014, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Abdollahi, S.; Salehi-Abargouei, A.; Toupchian, O.; Sheikhha, M.H.; Fallahzadeh, H.; Rahmanian, M.; Tabatabaie, M.; Mozaffari-Khosravi, H. The effect of resveratrol supplementation on cardio-metabolic risk factors in patients with type 2 diabetes: A randomized, double-blind controlled trial. Phytother. Res. 2019, 33, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimaraes, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves metabolic syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, A.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Effect of resveratrol on blood lipid levels in patients with type 2 diabetes: A systematic review and meta-analysis. Obesity 2019, 27, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, T.M.; Ogawa, L.Y.; Rogero, M.M.; Barroso, L.P.; Alves de Castro, I. Effect of resveratrol supplementation on biomarkers associated with atherosclerosis in humans. Complement. Ther. Clin. Pract. 2022, 46, 101491. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef]
- Akbari, M.; Tamtaji, O.R.; Lankarani, K.B.; Tabrizi, R.; Dadgostar, E.; Haghighat, N.; Kolahdooz, F.; Ghaderi, A.; Mansournia, M.A.; Asemi, Z. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Lipids. Health Dis. 2020, 19, 25. [Google Scholar] [CrossRef] [Green Version]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—Systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Melgar, A.; Izquierdo-Ramírez, P.J.; Griñán-Ferré, C.; Pallàs, M.; Martín, M.; Albasanz, J.L. Neuroprotective Effects of Resveratrol by Modifying Cholesterol Metabolism and Aβ Processing in SAMP8 Mice. Int. J. Mol. Sci. 2022, 23, 7580. https://doi.org/10.3390/ijms23147580
Sánchez-Melgar A, Izquierdo-Ramírez PJ, Griñán-Ferré C, Pallàs M, Martín M, Albasanz JL. Neuroprotective Effects of Resveratrol by Modifying Cholesterol Metabolism and Aβ Processing in SAMP8 Mice. International Journal of Molecular Sciences. 2022; 23(14):7580. https://doi.org/10.3390/ijms23147580
Chicago/Turabian StyleSánchez-Melgar, Alejandro, Pedro J. Izquierdo-Ramírez, Christian Griñán-Ferré, Mercè Pallàs, Mairena Martín, and José Luis Albasanz. 2022. "Neuroprotective Effects of Resveratrol by Modifying Cholesterol Metabolism and Aβ Processing in SAMP8 Mice" International Journal of Molecular Sciences 23, no. 14: 7580. https://doi.org/10.3390/ijms23147580