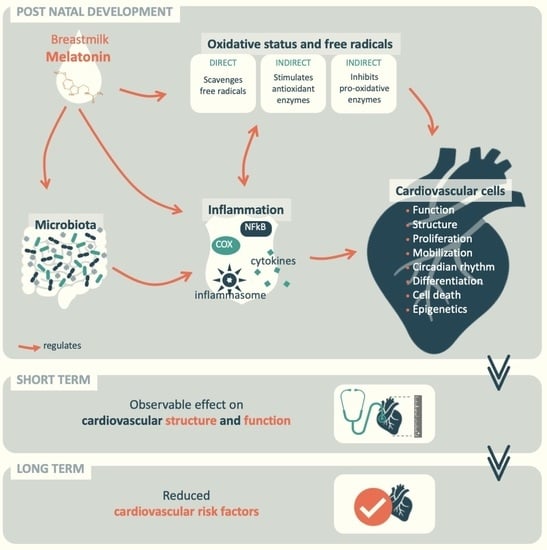
Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System
Abstract
:1. Introduction
1.1. Early Nutrition and Health Programming
1.2. Melatonin: The Circadian Hormone
- When present at the surface of a cell, melatonin binds to specific G protein-coupled receptors named MT1 and MT2 [31], which are 350 and 363 amino acids long, respectively, and they are associated with proteins Gi and Gq. Melatonin then regulates the activity of adenylate cyclase, phospholipases C and A2, potassium and calcium channels, and guanylyl cyclase. These receptors are both present in the cardiovascular system, in which MT1 has been observed to mediate vascular constriction and MT2 vasodilatation. Their repartition changes in the vascular tree, explaining why melatonin has been reported as a vasoconstrictor in caudal arteries but a vasorelaxant in the aorta and mesenteric artery [31]. Melatonin also has an action on autophagy, which has been shown to be mediated by the α-7 nicotinic receptors [32].
- Another pathway of action of melatonin occurs through reaching and binding nuclear receptors inside the cell and regulating transcription. The subfamilies of the nuclear receptors retinoid Z receptor/retinoid receptor-related orphan receptor, RZRa, RORa, RORa2, and RZRb reportedly have their activity regulated by melatonin, modulating the expression of their target genes [31]. For instance, following melatonin stimulation, RZR/RORa has been shown to bind DNA, increasing the transcription of the mRNA coding for ɣ-glutamylcysteine synthetase (ɣ-GCS), which is the limiting enzyme of glutathione (GSH) synthesis. Consequently, the enzyme and GSH are expressed to protect the cell from oxidative stress and to regulate the cell cycle [33]. It is believed that through these membrane and nuclear receptors, melatonin triggers clock gene expression by the direct circadian regulation of cellular pathways in both nucleated and non-nucleated cells. Melatonin also synchronizes the “peripheral clocks,” circadian oscillators present in all nucleated cells that ensure the presence of circadian rhythms of the organism even in the absence of an external synchronizer. They are composed of a group of genes known as “clock genes,” the circadian locomotor output cycles kaput (Clock), brain and muscle ARNT-like protein-1 (Bmal1), Period (Per), and Cryptochrome (Cry) genes, together forming a multiple loop of regulation with a period of approximately twenty-four hours. Under their control are “clock-controlled genes,” an important proportion of the genes involved in metabolism and inflammation, such as peroxisome proliferator-activated receptor γ (PPARγ), chemokine (C-C motif) ligand 2 (Ccl2), hypoxia-inducible factor (HIF)-1α, or glucose transporter 1 (Glut-1) [34]. For example, in adipose tissue, melatonin regulates the differentiation of adipocytes and the expression of clock genes (Clock, Per1) that increase the expression of their mRNA at night. In this way, melatonin also entrains the circadian rhythms of lipogenesis and lipolysis and fatty acid and glucose uptake [35,36,37].
- In addition to regulating signals via receptors, melatonin activity is mediated by low-affinity interactions with proteins, such as enzymes, transporters, calcium-binding proteins, and cytoskeletal and scaffold proteins, both in the cytosol and organelles such as the mitochondria. Through its interaction with the Ca+-calmodulin complex, melatonin influences the formation of microtubules and has also been associated with endothelial nitric oxide synthase (eNOS) inhibition, triggering vasoconstriction [38]. Furthermore, by acting indirectly via its modulation of cellular oxidative stress, melatonin has been reported to regulate cellular pathways involving extracellular signal-regulated kinases (ERK1/2), protein kinase B (Akt), protein kinase C (PKC), and NFkB activation, leading to the regulation of cell proliferation [39].
- Lastly, due to its biochemical structure, melatonin has the capacity to scavenge free radicals and limit oxidative stress inside cells [40].
2. Melatonin in Circadian Rhythms, in Relation to Cardiovascular Health
2.1. Melatonin and Circadian Rhythms
2.2. Circadian Rhythms and Cardiovascular Disorders
3. Melatonin in Oxidative Stress and Inflammation in Relation to Cardiovascular Health
3.1. Melatonin in Oxidative Stress and Inflammation
- The structure of melatonin confers great scavenging capacity. The core is an electron-rich indole heterocycle that has high resonance. This core is the primary agent of the antioxidant action but is not the only one. The methoxy and amide side chains and the carbonyl group of the N-C = O structure in the C3 amide side chain provide melatonin with the capacity to scavenge more free radicals. This cascade is a biochemical characteristic of the melatonin molecule that other antioxidants, such as vitamin C, E, or glutathione, do not possess [77].
- Furthermore, the biochemical structure is not the only way melatonin participates in reducing oxidative stress in the cell. In fact, the antioxidant capacity of melatonin also lies in the stimulation of enzymes involved in cellular antioxidant activity. This was evidenced by its stimulation of the genetic expression and enzymatic activities of glutathione peroxidase, glutathione reductase, superoxide dismutase, and catalase. Through glutathione reductase stimulation, melatonin helps to ensure a high GSH:GSSG ratio [78]. Additionally, at least in some human cells, melatonin can increase the ɣ-glutamyl-cysteine synthetase expression, allowing the de novo expression of glutathione [33].
- Melatonin is involved in a third pathway that regulates oxidation by limiting the production of oxidants in the cell. It regulates the expression of the inducible nitric oxide synthase (iNOS). By lowering the expression of this enzyme, melatonin can indirectly prevent oxidative and nitrosative damage [38,79,80].
3.2. Oxidative Stress, Inflammation, and Cardiovascular Health
4. Melatonin in Oxidative Stress and Inflammation in Relation to Cardiovascular Health
4.1. Melatonin in Gut Microbiota Regulation
- (1)
- An extensive number of gut bacterial genera and species, as well as the microbial community, exhibit oscillatory behavior. The circadian rhythms of the gut microbiota are a feature of the physiology of a healthy gut microbiota, presenting daily fluctuations in community populations observable by changes in the most frequently represented bacterial strains, as well as circadian fluctuations in bacterial function and metabolite expression [99]. A clear association has been observed between dysbiosis and alterations in the circadian rhythms of the gut microbiota induced by nutritional factors and host metabolic disorders. In high-fat diet-fed mice (HFD), antibiotic treatment disturbed the gut microbiota and promoted metabolic impairment that improves after melatonin administration [100,101]. The gut microbiota of humans with circadian disruption secondary to jet lag, when transplanted into germ-free mice, induced obesity and glucose intolerance, an effect that was not observed when inoculating microbiota from non-jet-lagged individuals [102]. Melatonin has the capacity to reestablish the balance of GI microbiota by promoting the growth of Alistipes and Bacteroides, which are beneficial bacteria in the GI tract [103]. Thus, melatonin significantly improved metabolic disturbances, restoring healthy microbiota and circadian rhythms. The rhythmic expression of melatonin reaching the lumen of the gastrointestinal tract participates in synchronizing the circadian rhythms of the gut microbiota. For example, the gut bacteria Enterobacter aerogenes was observed responding to melatonin from the pineal gland and from the gastrointestinal tract by increasing its swarming activity [104]. Melatonin has the ability to decrease the Firmicutes-to-Bacteroidetes ratio in rodents and increase the abundance of mucin-degrading bacteria Akkermansia, which is associated with healthy mucosa [105]. The consequent disruptions to microbiota-mediated functions, such as the decreased conjugation of bile acids or increased production of hydrogen sulfide and the resulting decreased production of butyrate, in turn affect substrate oxidation and energy regulation in the host. Thus, disturbances in microbiome rhythms may at least partially contribute to an increased risk of obesity and metabolic syndrome associated with insufficient sleep and circadian misalignment. It is therefore likely that the rhythmic melatonin levels in breast milk have this effect on the newborn’s symbionts when they do not yet produce their own rhythmic melatonin.
- (2)
- In addition, the action of melatonin on the gut microbiota and its interaction with the host physiology may be mediated by another pathway: the regulation of oxidative stress/inflammation. Melatonin is secreted by enterochromaffin cells in high quantities. In fact, the melatonin generated in the gastrointestinal tract surpasses pineal melatonin by 10–100 fold. It was speculated that most of the circulatory melatonin during the day was derived from the gastrointestinal tract. Its potent antioxidant activity contributes to maintaining the integrity of the intestinal barrier [106]. This barrier is composed of physical constraints, such as polymers of mucins limiting access to the epithelium, and numerous factors from the immune system ensuring the protection of the barrier, displaying circadian rhythms under physiological conditions [92,93]. The primary mechanisms by which melatonin is involved in this action are to regulate the abundance of matrix metalloproteinases, regulate immunological damage by the activity of macrophages, decrease oxidative stress, inhibit the production of nitric oxide, suppress the activity of NF-kB, and decrease the level of cytokines that promote inflammation [73].
4.2. Gut Microbiota Regulation and Cardiovascular Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RZR/RORa | Retinoid Z receptor/retinoid receptor-related orphan receptor a |
| ɣ-GCS | ɣ-glutamylcysteine synthetase |
| GSH | Glutathione |
| Clock | Circadian locomotor output cycles kaput |
| Bmal1 | Brain and muscle ARNT-like protein-1 |
| Per | Period |
| Cry | Cryptochrome |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| Ccl2 | Chemokine (C-C motif) ligand 2 |
| (HIF)-1α | Hypoxia-inducible factor |
| Glut-1 | Glucose transporter 1 |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| eNOS | Endothelial nitric oxide synthase |
| ERK1/2 | Extracellular signal-regulated kinases |
| Akt | Protein kinase B |
| PKC | Protein kinase C |
| iNOS | Inducible nitric oxide synthase |
| MAPK | Mitogen-activated protein kinases |
| HFD | High-fat diet |
References
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indrio, F.; Martini, S.; Francavilla, R.; Corvaglia, L.; Cristofori, F.; Mastrolia, S.A.; Neu, J.; Rautava, S.; Spena, G.R.; Raimondi, F.; et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front. Pediatr. 2017, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martín-Cabrejas, M.A.; De Pablo, Á.L.; De Pipaón, M.S.; Ramiro-Cortijo, D. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef] [Green Version]
- Binns, C.W.; Lee, M.K. Exclusive breastfeeding for six months: The WHO six months recommendation in the Asia Pacific Region. Asia Pac. J. Clin. Nutr. 2014, 23, 23. [Google Scholar]
- Shields, L.; Mamun, A.A.; O’Callaghan, M.; Williams, G.M.; Najman, J.M. Breastfeeding and obesity at 21 years: A cohort study: Breastfeeding and obesity at 21 years. J. Clin. Nurs. 2010, 19, 1612–1617. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. Breastfeeding and obesity at 21 years: A cohort study. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Evelein, A.M.; Geerts, C.C.; Visseren, F.L.; Bots, M.L.; Van Der Ent, C.K.; Grobbee, D.E.; Uiterwaal, C.S. The association between breastfeeding and the cardiovascular system in early childhood. Am. J. Clin. Nutr. 2011, 93, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.I.; Hwang, S.-J.; Ingelsson, E.; Benjamin, E.J.; Fox, C.S.; Vasan, R.S.; Murabito, J.M. Breastfeeding in Infancy and Adult Cardiovascular Disease Risk Factors. Am. J. Med. 2009, 122, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Barker, D. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Italianer, M.; Naninck, E.; Roelants, J.; Van Der Horst, G.; Reiss, I.; Goudoever, J.; Joosten, K.; Chaves, I.; Vermeulen, M. Circadian Variation in Human Milk Composition, a Systematic Review. Nutrients 2020, 12, 2328. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.L.; Cubero, J.; Sánchez, J.; Chanclón, B.; Rivero, M.; Rodríguez, A.B.; Barriga, C. The possible role of human milk nucleotides as sleep inducers. Nutr. Neurosci. 2009, 12, 2–8. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.R.; Gangur, V. Review: Immune Markers in Breast Milk and Fetal and Maternal Body Fluids: A Systematic Review of Perinatal Concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Billard, H.; Boquien, C.-Y.; Joram-Gauvard, E.; Simon, L.; Legrand, A.; Boscher, C.; Rozé, J.-C.; Jimenez, F.J.B.; Kaeffer, B. MiRNA Analysis by Quantitative PCR in Preterm Human Breast Milk Reveals Daily Fluctuations of hsa-miR-16-5p. PLoS ONE 2015, 10, e0140488. [Google Scholar] [CrossRef] [Green Version]
- Hahn-Holbrook, J.; Saxbe, D.; Bixby, C.; Steele, C.; Glynn, L. Human milk as “chrononutrition”: Implications for child health and development. Pediatr. Res. 2019, 85, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Schomerus, C. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef]
- McCarthy, R.; Jungheim, E.S.; Fay, J.C.; Bates, K.; Herzog, E.D.; England, S.K. Riding the Rhythm of Melatonin Through Pregnancy to Deliver on Time. Front. Endocrinol. 2019, 10, 616. [Google Scholar] [CrossRef] [Green Version]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular Benefits of Dietary Melatonin: A Myth or a Reality? Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, Á.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of Melatonin Supplementation in Women with Unexplained Infertility Undergoing Fertility Treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.; Vaillancourt, C.; Maes, M.; Reiter, R.J. Breast Feeding and Melatonin: Implications for Improving Perinatal Health. J. Breastfeed. Biol. 2015, 1, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, N.L.; Maloney, S.K.; Pillow, J.J.; Mark, P.J. Endocrine consequences of circadian rhythm disruption in early life. Curr. Opin. Endocr. Metab. Res. 2020, 11, 65–71. [Google Scholar] [CrossRef]
- Mirmiran, M.; Maas, Y.G.; Ariagno, R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003, 7, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparici-Gonzalo, S.; Carrasco-García, Á.; Gombert, M.; Carrasco-Luna, J.; Pin-Arboledas, G.; Codoñer-Franch, P. Melatonin Content of Human Milk: The Effect of Mode of Delivery. Breastfeed. Med. 2020, 15, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.A. Infant Sleep and Feeding. J. Obstet. Gynecol. Neonatal Nurs. 2008, 37, 706–714. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Casado, M.E.D.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- McKenna, H.; Reiss, I.K.M. The case for a chronobiological approach to neonatal care. Early Hum. Dev. 2018, 126, 1–5. [Google Scholar] [CrossRef]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm Heart in Adult Life. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Shi, W.; Zhuang, J.; Liu, Y.; Tang, L.; Bu, J.; Sun, J.; Bei, F. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep. 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; De Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayo, J.C.; Sainz, R.M.; González-Menéndez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin transport into mitochondria. Cell. Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-K.; Park, S.-Y. Melatonin regulates the autophagic flux via activation of alpha-7 nicotinic acetylcholine receptors. J. Pineal Res. 2015, 59, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free. Radic. Biol. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Vale, M.I.; Andreotti, S.; Mukai, P.Y.; Borges-Silva, C.D.N.; Peres, S.B.; Cipolla-Neto, J.; Lima, F. Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J. Pineal Res. 2008, 45, 422–429. [Google Scholar] [CrossRef]
- Man, A.; Xia, N.; Li, H. Circadian Rhythm in Adipose Tissue: Novel Antioxidant Target for Metabolic and Cardiovascular Diseases. Antioxidants 2020, 9, 968. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and Central Effects of Melatonin on Blood Pressure Regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef] [Green Version]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J. 2010, 24, 3603–3624. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2006, 42, 28–42. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Cubero, J.; Valero, V.; Sánchez, J.; Rivero, M.; Parvez, H.; Rodríguez, A.B.; Barriga, C. The circadian rhythm of tryptophan in breast milk affects the rhythms of 6-sulfatoxymelatonin and sleep in newborn. Neuro Endocrinol. Lett. 2005, 26, 657–661. [Google Scholar]
- Fernandez, A.; Ordóñez, R.; Reiter, R.J.; González-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Hartikainen, J.; Tarkiainen, I.; Tahvanainen, K.; Mäntysaari, M.; Länsimies, E.; Pyörälä, K. Circadian variation of cardiac autonomic regulation during 24-h bed rest. Clin. Physiol. 1993, 13, 185–196. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K. Circadian clock and the onset of cardiovascular events. Hypertens. Res. 2016, 39, 383–390. [Google Scholar] [CrossRef]
- Giles, T.D. Circadian rhythm of blood pressure and the relation to cardiovascular events. J. Hypertens. 2006, 24, S11–S16. [Google Scholar] [CrossRef]
- Maemura, K.; Takeda, N.; Nagai, R. Circadian rhythms in the CNS and peripheral clock disorders: Role of the biological clock in cardiovascular diseases. J. Pharmacol. Sci. 2007, 103, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-F.; Stein, P.K. Circadian rhythm in the cardiovascular system: Chronocardiology. Am. Hear. J. 2003, 145, 779–786. [Google Scholar] [CrossRef]
- Aguilar-Sánchez, C.; Michael, M.; Pennings, S. Cardiac Stem Cells in the Postnatal Heart: Lessons from Development. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Huang, L.-T.; Hsu, C.-N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Lin, J.; Wang, S.; Cheng, Z.; Hu, J.; Wang, T.; Man, W.; Yin, T.; Guo, W.; Gao, E.; et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J. Pineal Res. 2017, 63, e12418. [Google Scholar] [CrossRef] [PubMed]
- Massin, M.M.; Maeyns, K.; Withofs, N.; Ravet, F.; Gérard, P.; Healy, M.J.R. Circadian rhythm of heart rate and heart rate variability. Arch. Dis. Child. 2000, 83, 179–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, C.J. Human circadian rhythms in heart rate response to a maximal exercise stress. Ergonomics 1980, 23, 591–595. [Google Scholar] [CrossRef]
- Tsimakouridze, E.V.; Alibhai, F.J.; Martino, T.A. Therapeutic applications of circadian rhythms for the cardiovascular system. Front. Pharmacol. 2015, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- Tarquini, R.; Mazzoccoli, G. Clock Genes, Metabolism, and Cardiovascular Risk. Hear. Fail. Clin. 2017, 13, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef] [Green Version]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mirick, D.K.; Chen, C.; Stanczyk, F.Z. Night Shift Work and Hormone Levels in Women. Cancer Epidemiol. Biomark. Prev. 2012, 21, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Peplonska, B.; Bukowska, A.; Sobala, W. Association of Rotating Night Shift Work with BMI and Abdominal Obesity among Nurses and Midwives. PLoS ONE 2015, 10, e0133761. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Castriotta, R.; Portaluppi, F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007, 8, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Hu, K.; Scheer, F.A.J.L. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J. Biol. Rhythm. 2017, 32, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.J.; Durrington, H.; Gibbs, J.E.; Blaikley, J.; Loudon, A.; Ray, D.W.; Sabroe, I. A matter of time: Study of circadian clocks and their role in inflammation. J. Leukoc. Biol. 2016, 99, 549–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Solera, M.; Carrasco-Luna, J.; Pin-Arboledas, G.; González-Carrascosa, R.; Soriano, J.; Codoñer-Franch, P. Short Sleep Duration Is Related to Emerging Cardiovascular Risk Factors in Obese Children. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 571–576. [Google Scholar] [CrossRef]
- Chan, J.Y.S.; Li, A.M.; Au, C.-T.; Lo, A.F.C.; Ng, S.-K.; Abdullah, V.J.; Ho, C.; Yu, C.-M.; Fok, T.-F.; Wing, Y.K. Cardiac remodelling and dysfunction in children with obstructive sleep apnoea: A community based study. Thorax 2009, 64, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, D.; Li, X.; Rodriguez-Colon, S.M.; Liu, J.; Vgontzas, A.N.; Calhoun, S.; Bixler, E.O. Sleep-disordered breathing and cardiac autonomic modulation in children. Sleep Med. 2010, 11, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Amin, R.; Somers, V.K.; McConnell, K.; Willging, P.; Myer, C.; Sherman, M.; McPhail, G.; Morgenthal, A.; Fenchel, M.; Bean, J.; et al. Activity-Adjusted 24-Hour Ambulatory Blood Pressure and Cardiac Remodeling in Children with Sleep Disordered Breathing. Hypertension 2008, 51, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, S.; Nishihara, K.; Horiuchi, S.; Eto, H. The influence of feeding method on a mother’s circadian rhythm and on the development of her infant’s circadian rest-activity rhythm. Early Hum. Dev. 2020, 145, 105046. [Google Scholar] [CrossRef]
- Engler, A.C.; Hadash, A.; Shehadeh, N.; Pillar, G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. Eur. J. Nucl. Med. Mol. Imaging 2011, 171, 729–732. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Bertagnolli, M.; Lukaszewski, M.-A.; Huyard, F.; Yzydorczyk, C.; Luu, T.M.; Nuyt, A.M. Preterm Birth and Hypertension Risk. Hypertension 2014, 63, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zores-Koenig, C.; Kuhn, P.; Caeymaex, L.; Allen, A.; Berne-Audeoud, F.; Bouvard, C.; Brandicourt, A.; Casper, C.; Denoual, H.; Duboz, M.A.; et al. Recommendations on neonatal light environment from the French Neonatal Society. Acta Paediatr. 2020, 109, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol. Life. Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef]
- Johns, J.R.; Platts, J.A. Theoretical insight into the antioxidant properties of melatonin and derivatives. Org. Biomol. Chem. 2014, 12, 7820–7827. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Qi, W. Biochemical Reactivity of Melatonin with Reactive Oxygen and Nitrogen Species: A Review of the Evidence. Cell Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Tan, D.-X.; Reiter, R.; Manchester, L.; Yan, M.-T.; El-Sawi, M.; Sainz, R.; Mayo, J.; Kohen, R.; Allegra, M.; Hardelan, R. Chemical and Physical Properties and Potential Mechanisms: Melatonin as a Broad Spectrum Antioxidant and Free Radical Scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.-X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [Green Version]
- Carnicer, R.; Crabtree, M.J.; Sivakumaran, V.; Casadei, B.; Kass, D.A. Nitric Oxide Synthases in Heart Failure. Antioxid. Redox Signal. 2013, 18, 1078–1099. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.P.; Al-Hasan, Y. Impact of Oxidative Stress in Fetal Programming. J. Pregnancy 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Cooper, D.M.; Radom-Aizik, S. Exercise-associated prevention of adult cardiovascular disease in children and adolescents: Monocytes, molecular mechanisms, and a call for discovery. Pediatr. Res. 2019, 87, 309–318. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Reiter, R.J.; Rikhtegar, R.; Jalili, J.; Hajalioghli, P.; Mihanfar, A.; Majidinia, M.; Yousefi, B. Melatonin: An atypical hormone with major functions in the regulation of angiogenesis. IUBMB Life 2020, 72, 1560–1584. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Franco, C.; Stacchiotti, A.; Rodella, L.F.; Rezzani, R. Sirtuin1 Role in the Melatonin Protective Effects Against Obesity-Related Heart Injury. Front. Physiol. 2020, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Ostadmohammadi, V.; Mirhosseini, N.; Lankarani, K.B.; Tabrizi, R.; Keshtkaran, Z.; Reiter, R.J.; Asemi, Z. The effects of melatonin supplementation on blood pressure in patients with metabolic disorders: A systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens. 2019, 33, 202–209. [Google Scholar] [CrossRef]
- Benova, T.E.; Viczenczova, C.; Bacova, B.S.; Knezl, V.; Dosenko, V.; Rauchova, H.; Zeman, M.; Reiter, R.J.; Tribulova, N. Obesity-associated alterations in cardiac connexin-43 and PKC signaling are attenuated by melatonin and omega-3 fatty acids in female rats. Mol. Cell. Biochem. 2019, 454, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Reiter, R.J.; Ahmad, N. Sirtuins, melatonin and circadian rhythms: Building a bridge between aging and cancer. J. Pineal Res. 2009, 48, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Gombert, M. Circadian rhythms in the pathogenesis of gastrointestinal diseases. World J. Gastroenterol. 2018, 24, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Gombert, M.; Carrasco-Luna, J.; Pin-Arboledas, G.; Codoñer-Franch, P. The connection of circadian rhythm to inflammatory bowel disease. Transl. Res. 2019, 206, 107–118. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.A.; Thorsen, J.; Dominguez-Bello, M.G.; Blaser, M.J.; Mortensen, M.S.; Brejnrod, A.D.; Shah, S.A.; Hjelmsø, M.H.; Lehtimäki, J.; Trivedi, U.; et al. Ecological succession in the vaginal microbiota during pregnancy and birth. ISME J. 2020, 14, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Voigt, R.; Forsyth, C.; Green, S.; Engen, P.; Keshavarzian, A. Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 131, 193–205. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. Msystems 2020, 5. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulose, J.K.; Cassone, V.M. The melatonin-sensitive circadian clock of the enteric bacteriumEnterobacter aerogenes. Gut Microbes 2016, 7, 424–427. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Esteban-Zubero, E.; López-Pingarrón, L.; Alatorre-Jiménez, M.A.; Ochoa-Moneo, P.; Buisac-Ramón, C.; Rivas-Jiménez, M.; Castán-Ruiz, S.; Antoñanzas-Lombarte, Á.; Tan, D.-X.; García, J.J.; et al. Melatonin’s role as a co-adjuvant treatment in colonic diseases: A review. Life Sci. 2017, 170, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.H.; Gøbel, R.J.; Hansen, T.; Pedersen, O. The gut microbiome in cardio-metabolic health. Genome Med. 2015, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Asmakh, M.; Zadjali, F. Use of Germ-Free Animal Models in Microbiota-Related Research. J. Microbiol. Biotechnol. 2015, 25, 1583–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ney, D.M.; Murali, S.G.; Stroup, B.M.; Nair, N.; Sawin, E.A.; Rohr, F.; Levy, H.L. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol. Genet. Metab. 2017, 121, 96–103. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombert, M.; Codoñer-Franch, P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. Int. J. Mol. Sci. 2021, 22, 6809. https://doi.org/10.3390/ijms22136809
Gombert M, Codoñer-Franch P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. International Journal of Molecular Sciences. 2021; 22(13):6809. https://doi.org/10.3390/ijms22136809
Chicago/Turabian StyleGombert, Marie, and Pilar Codoñer-Franch. 2021. "Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System" International Journal of Molecular Sciences 22, no. 13: 6809. https://doi.org/10.3390/ijms22136809