Neural Stem Cell Transplantation for Neurodegenerative Diseases
Abstract
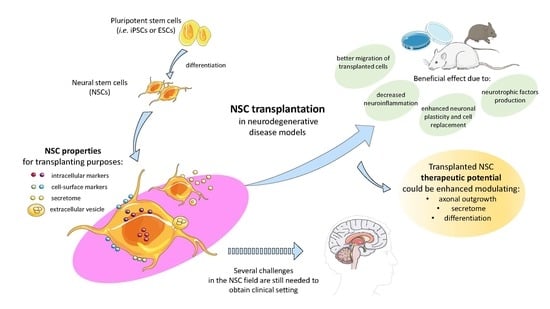
:1. Introduction
2. NSC Properties for Transplanting Purposes
2.1. NSC Markers and Subtypes
2.2. NSC Secretome
2.3. NSC Extracellular Vesicles
3. Migration of Transplanted NSCs
4. Enhancing the Therapeutic Potential of NSCs in Neurodegenerative Diseases
4.1. Modulation of Axonal Outgrowth
4.2. Modulation of the NSC Secretome
4.3. Modulation of NSC Differentiation and Functional Properties
5. Requirements and Feature Assessment for Clinical Grade iPSCs
6. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Heemels, M.-T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkle, F.T.; Alvarez-Buylla, A. Neural stem cells in mammalian development. Curr. Opin. Cell Biol. 2006, 18, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.A.; Palmer, T.D. Immune influence on adult neural stem cell regulation and function. Neuron 2009, 64, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, R.J.M.; Ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Central Nervous System. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Tuazon, J.P.; Castelli, V.; Lee, J.-Y.; Desideri, G.B.; Stuppia, L.; Cimini, A.M.; Borlongan, C.V. Neural Stem Cells. Adv. Exp. Med. Biol. 2019, 1201, 79–91. [Google Scholar] [CrossRef]
- Forotti, G.; Nizzardo, M.; Bucchia, M.; Ramirez, A.; Trombetta, E.; Gatti, S.; Bresolin, N.; Comi, G.P.; Corti, S. CSF transplantation of a specific iPSC-derived neural stem cell subpopulation ameliorates the disease phenotype in a mouse model of spinal muscular atrophy with respiratory distress type 1. Exp. Neurol. 2019, 321, 113041. [Google Scholar] [CrossRef]
- Nizzardo, M.; Bucchia, M.; Ramirez, A.; Trombetta, E.; Bresolin, N.; Comi, G.P.; Corti, S. iPSC-derived LewisX+CXCR4+β1-integrin+ neural stem cells improve the amyotrophic lateral sclerosis phenotype by preserving motor neurons and muscle innervation in human and rodent models. Hum. Mol. Genet. 2016, 25, 3152–3163. [Google Scholar] [CrossRef]
- Nizzardo, M.; Simone, C.; Rizzo, F.; Ruggieri, M.; Salani, S.; Riboldi, G.; Faravelli, I.; Zanetta, C.; Bresolin, N.; Comi, G.P.; et al. Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum. Mol. Genet. 2014, 23, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Simone, C.; Nizzardo, M.; Rizzo, F.; Ruggieri, M.; Riboldi, G.; Salani, S.; Bucchia, M.; Bresolin, N.; Comi, G.P.; Corti, S. iPSC-Derived neural stem cells act via kinase inhibition to exert neuroprotective effects in spinal muscular atrophy with respiratory distress type 1. Stem Cell Rep. 2014, 3, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-S.; Jung, K.; Kim, I.-S.; Lee, H.; Kim, M.; Yun, S.; Hwang, K.; Shin, J.E.; Park, K.I. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 2015, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Manadas, B.; Behie, L.A.; Salgado, A.J. Secretome of Undifferentiated Neural Progenitor Cells Induces Histological and Motor Improvements in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2018, 7, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucchia, M.; Ramirez, A.; Parente, V.; Simone, C.; Nizzardo, M.; Magri, F.; Dametti, S.; Corti, S. Therapeutic development in amyotrophic lateral sclerosis. Clin. Ther. 2015, 37, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, J.K.; Draper, J.S.; Baillie, H.S.; Fishel, S.; Thomson, J.A.; Moore, H.; Andrews, P.W. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells Dayt. Ohio 2002, 20, 329–337. [Google Scholar] [CrossRef]
- Pruszak, J.; Sonntag, K.-C.; Aung, M.H.; Sanchez-Pernaute, R.; Isacson, O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells Dayt. Ohio 2007, 25, 2257–2268. [Google Scholar] [CrossRef] [Green Version]
- Pruszak, J.; Ludwig, W.; Blak, A.; Alavian, K.; Isacson, O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells Dayt. Ohio 2009, 27, 2928–2940. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, G. The Effects of Different Factors on the Behavior of Neural Stem Cells. Available online: https://www.hindawi.com/journals/sci/2017/9497325/ (accessed on 14 April 2020).
- Tang, Y.; Yu, P.; Cheng, L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017, 8, e3108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, C.T.; Chen, J.; Pankratz, M.T.; Xi, J.; Li, J.; Yang, Y.; Lavaute, T.M.; Li, X.-J.; Ayala, M.; et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 2010, 7, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Jandial, R.; Singec, I.; Ames, C.P.; Snyder, E.Y. Genetic modification of neural stem cells. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 450–457. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. BioMed Res. Int. 2015, 2015, 727542. [Google Scholar] [CrossRef] [Green Version]
- Elkouris, M.; Balaskas, N.; Poulou, M.; Politis, P.K.; Panayiotou, E.; Malas, S.; Thomaidou, D.; Remboutsika, E. Sox1 maintains the undifferentiated state of cortical neural progenitor cells via the suppression of Prox1-mediated cell cycle exit and neurogenesis. Stem Cells Dayt. Ohio 2011, 29, 89–98. [Google Scholar] [CrossRef]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef]
- Park, D.; Xiang, A.P.; Mao, F.F.; Zhang, L.; Di, C.-G.; Liu, X.-M.; Shao, Y.; Ma, B.-F.; Lee, J.-H.; Ha, K.-S.; et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells Dayt. Ohio 2010, 28, 2162–2171. [Google Scholar] [CrossRef]
- Noisa, P.; Lund, C.; Kanduri, K.; Lund, R.; Lähdesmäki, H.; Lahesmaa, R.; Lundin, K.; Chokechuwattanalert, H.; Otonkoski, T.; Tuuri, T.; et al. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J. Cell Sci. 2014, 127, 2083–2094. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, R.; Ohtsuka, T.; Shimojo, H.; Imayoshi, I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat. Neurosci. 2008, 11, 1247–1251. [Google Scholar] [CrossRef]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A.; et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 2011, 6, e17540. [Google Scholar] [CrossRef] [Green Version]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef] [Green Version]
- Friocourt, G.; Koulakoff, A.; Chafey, P.; Boucher, D.; Fauchereau, F.; Chelly, J.; Francis, F. Doublecortin functions at the extremities of growing neuronal processes. Cereb. Cortex N. Y. N 1991 2003, 13, 620–626. [Google Scholar] [CrossRef]
- Gurok, U.; Steinhoff, C.; Lipkowitz, B.; Ropers, H.-H.; Scharff, C.; Nuber, U.A. Gene Expression Changes in the Course of Neural Progenitor Cell Differentiation. J. Neurosci. 2004, 24, 5982–6002. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Thomas, R.; Elgueta, C.; Horl, M.; Osborn, T.; Hallett, P.J.; Bartos, M.; Isacson, O.; Pruszak, J. Comprehensive Cell Surface Antigen Analysis Identifies Transferrin Receptor Protein-1 (CD71) as a Negative Selection Marker for Human Neuronal Cells. Stem Cells Dayt. Ohio 2019, 37, 1293–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turaç, G.; Hindley, C.J.; Thomas, R.; Davis, J.A.; Deleidi, M.; Gasser, T.; Karaöz, E.; Pruszak, J. Combined flow cytometric analysis of surface and intracellular antigens reveals surface molecule markers of human neuropoiesis. PLoS ONE 2013, 8, e68519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundberg, M.; Jansson, L.; Ketolainen, J.; Pihlajamäki, H.; Suuronen, R.; Skottman, H.; Inzunza, J.; Hovatta, O.; Narkilahti, S. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009, 2, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, R.H.; Fruttiger, M.; Richardson, W.D.; Franklin, R.J.M. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 2004, 25, 252–262. [Google Scholar] [CrossRef]
- Wright, G.J.; Cherwinski, H.; Foster-Cuevas, M.; Brooke, G.; Puklavec, M.J.; Bigler, M.; Song, Y.; Jenmalm, M.; Gorman, D.; McClanahan, T.; et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. Baltim. Md 1950 2003, 171, 3034–3046. [Google Scholar] [CrossRef] [Green Version]
- Gorczynski, R.; Chen, Z.; Kai, Y.; Lee, L.; Wong, S.; Marsden, P.A. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. Baltim. Md 1950 2004, 172, 7744–7749. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Hu, A.; Zhu, J.; Yu, J.; Talebian, F.; Bai, X.-F. CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy. Adv. Exp. Med. Biol. 2020, 1223, 155–165. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, M.; Chen, S.; Ding, J. CD200 Inhibits Inflammatory Response by Promoting KATP Channel Opening in Microglia Cells in Parkinson’s Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 1733–1741. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-J.; Ye, M.; Zhang, Y.-H.; Chen, S.-D. CD200-CD200R regulation of microglia activation in the pathogenesis of Parkinson’s disease. J. Neuroimmune Pharmacol. 2007, 2, 259–264. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, D.-X.; Kai, Y.; Khatri, I.; Lamptey, B.; Gorczynski, R. Identification of an Expressed Truncated Form of CD200, CD200tr, which is a Physiologic Antagonist of CD200-Induced Suppression. Transplantation 2008, 86, 1116–1124. [Google Scholar] [CrossRef]
- Varnum, M.M.; Kiyota, T.; Ingraham, K.L.; Ikezu, S.; Ikezu, T. The anti-inflammatory glycoprotein, cd200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of alzheimer’s disease. Neurobiol. Aging 2015, 36, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Li, J.; Sun, H. CD200-CD200R Interaction: An Important Regulator After Stroke. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Manich, G.; Recasens, M.; Valente, T.; Almolda, B.; González, B.; Castellano, B. Role of the CD200-CD200R Axis During Homeostasis and Neuroinflammation. Neuroscience 2019, 405, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Tanaka, N.; Oishi, Y.; Hamasaki, T.; Nakanishi, K.; Sakai, N.; Ochi, M. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine 2007, 32, 1272–1278. [Google Scholar] [CrossRef]
- Park, D.; Yang, Y.-H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.-K.; Lee, S.W.; Kim, G.H.; et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef]
- Waterhouse, E.G.; An, J.J.; Orefice, L.L.; Baydyuk, M.; Liao, G.-Y.; Zheng, K.; Lu, B.; Xu, B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J. Neurosci. 2012, 32, 14318–14330. [Google Scholar] [CrossRef]
- Ding, J.; He, Z.; Ruan, J.; Liu, Y.; Gong, C.; Sun, S.; Chen, H. Influence of endogenous ciliary neurotrophic factor on neural differentiation of adult rat hippocampal progenitors. Neural Regen. Res. 2013, 8, 301–312. [Google Scholar] [CrossRef]
- Hicks, C.; Stevanato, L.; Stroemer, R.P.; Tang, E.; Richardson, S.; Sinden, J.D. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013, 22, 1541–1552. [Google Scholar] [CrossRef]
- McGinley, L.M.; Sims, E.; Lunn, J.S.; Kashlan, O.N.; Chen, K.S.; Bruno, E.S.; Pacut, C.M.; Hazel, T.; Johe, K.; Sakowski, S.A.; et al. Human Cortical Neural Stem Cells Expressing Insulin-Like Growth Factor-I: A Novel Cellular Therapy for Alzheimer’s Disease. Stem Cells Transl. Med. 2016, 5, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Allodi, I.; Comley, L.; Nichterwitz, S.; Nizzardo, M.; Simone, C.; Benitez, J.A.; Cao, M.; Corti, S.; Hedlund, E. Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS. Sci. Rep. 2016, 6, 25960. [Google Scholar] [CrossRef] [Green Version]
- Pluchino, S.; Zanotti, L.; Rossi, B.; Brambilla, E.; Ottoboni, L.; Salani, G.; Martinello, M.; Cattalini, A.; Bergami, A.; Furlan, R.; et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005, 436, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Bacigaluppi, M.; Pluchino, S.; Peruzzotti-Jametti, L.; Jametti, L.P.; Kilic, E.; Kilic, U.; Salani, G.; Brambilla, E.; West, M.J.; Comi, G.; et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain J. Neurol. 2009, 132, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluchino, S.; Smith, J.A. Explicating Exosomes: Reclassifying the Rising Stars of Intercellular Communication. Cell 2019, 177, 225–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, C.M.; Nicaise, A.M.; Peruzzotti-Jametti, L.; Pluchino, S. The neural stem cell secretome and its role in brain repair. Brain Res. 2020, 1729, 146615. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, P.; Hazel, T.; Johe, K.; Koliatsos, V.E. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci. Lett. 2011, 494, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Corti, S.; Locatelli, F.; Papadimitriou, D.; Del Bo, R.; Nizzardo, M.; Nardini, M.; Donadoni, C.; Salani, S.; Fortunato, F.; Strazzer, S.; et al. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain J. Neurol. 2007, 130, 1289–1305. [Google Scholar] [CrossRef] [Green Version]
- Yabe, T.; Sanagi, T.; Yamada, H. The neuroprotective role of PEDF: Implication for the therapy of neurological disorders. Curr. Mol. Med. 2010, 10, 259–266. [Google Scholar] [CrossRef]
- Kajitani, K.; Nomaru, H.; Ifuku, M.; Yutsudo, N.; Dan, Y.; Miura, T.; Tsuchimoto, D.; Sakumi, K.; Kadoya, T.; Horie, H.; et al. Galectin-1 promotes basal and kainate-induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death Differ. 2009, 16, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, S.; Kaur, G.; Mi, W.; Tizon, B.; Levy, E. Protective mechanisms by cystatin C in neurodegenerative diseases. Front. Biosci. Sch. Ed. 2011, 3, 541–554. [Google Scholar]
- Wicher, G.; Fex-Svenningsen, Å.; Velsecchi, I.; Charnay, Y.; Aldskogius, H. Extracellular clusterin promotes neuronal network complexity in vitro. NeuroReport 2008, 19, 1487–1491. [Google Scholar] [CrossRef]
- Pasterkamp, R.J.; Peschon, J.J.; Spriggs, M.K.; Kolodkin, A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003, 424, 398–405. [Google Scholar] [CrossRef]
- Zuo, T.; Qin, J.Y.; Chen, J.; Shi, Z.; Liu, M.; Gao, X.; Gao, D. Involvement of N-cadherin in the protective effect of glial cell line-derived neurotrophic factor on dopaminergic neuron damage. Int. J. Mol. Med. 2013, 31, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Boulis, N.M.; Federici, T.; Glass, J.D.; Lunn, J.S.; Sakowski, S.A.; Feldman, E.L. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 8, 172–176. [Google Scholar] [CrossRef]
- Teng, Y.D.; Benn, S.C.; Kalkanis, S.N.; Shefner, J.M.; Onario, R.C.; Cheng, B.; Lachyankar, M.B.; Marconi, M.; Li, J.; Yu, D.; et al. Multimodal actions of neural stem cells in a mouse model of ALS: A meta-analysis. Sci. Transl. Med. 2012, 4, 165ra164. [Google Scholar] [CrossRef]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A.; Upadhya, R.; Shetty, A.K. Neural stem cell derived extracellular vesicles: Attributes and prospects for treating neurodegenerative disorders. EBioMedicine 2018, 38, 273–282. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Cantaluppi, V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem. Soc. Trans. 2013, 41, 283–287. [Google Scholar] [CrossRef]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Webb, R.L.; Kaiser, E.E.; Jurgielewicz, B.J.; Spellicy, S.; Scoville, S.L.; Thompson, T.A.; Swetenburg, R.L.; Hess, D.C.; West, F.D.; Stice, S.L. Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke 2018, 49, 1248–1256. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef]
- Fricker, R.A.; Carpenter, M.K.; Winkler, C.; Greco, C.; Gates, M.A.; Björklund, A. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 1999, 19, 5990–6005. [Google Scholar] [CrossRef] [Green Version]
- Gebara, E.; Bonaguidi, M.A.; Beckervordersandforth, R.; Sultan, S.; Udry, F.; Gijs, P.-J.; Lie, D.C.; Ming, G.-L.; Song, H.; Toni, N. Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells Dayt. Ohio 2016, 34, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.W.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566–580. [Google Scholar] [CrossRef] [Green Version]
- Rakic, P. Elusive radial glial cells: Historical and evolutionary perspective. Glia 2003, 43, 19–32. [Google Scholar] [CrossRef]
- Ladewig, J.; Koch, P.; Brüstle, O. Auto-attraction of neural precursors and their neuronal progeny impairs neuronal migration. Nat. Neurosci. 2014, 17, 24–26. [Google Scholar] [CrossRef]
- Gonzalez, R.; Hamblin, M.H.; Lee, J.-P. Neural Stem Cell Transplantation and CNS Diseases. CNS Neurol. Disord. Drug Targets 2016, 15, 881–886. [Google Scholar] [CrossRef]
- Ganat, Y.M.; Calder, E.L.; Kriks, S.; Nelander, J.; Tu, E.Y.; Jia, F.; Battista, D.; Harrison, N.; Parmar, M.; Tomishima, M.J.; et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J. Clin. Invest. 2012, 122, 2928–2939. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.L.; Anandakumaran, P.N.; Varga, B.V.; Morshead, C.M.; Nagy, A.; Shoichet, M.S. In Vitro Maturation of Human iPSC-Derived Neuroepithelial Cells Influences Transplant Survival in the Stroke-Injured Rat Brain. Tissue Eng. Part A 2018, 24, 351–360. [Google Scholar] [CrossRef]
- Linaro, D.; Vermaercke, B.; Iwata, R.; Ramaswamy, A.; Libé-Philippot, B.; Boubakar, L.; Davis, B.A.; Wierda, K.; Davie, K.; Poovathingal, S.; et al. Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits. Neuron 2019, 104, 972–986. [Google Scholar] [CrossRef] [Green Version]
- Barry, D.S.; Pakan, J.M.P.; McDermott, K.W. Radial glial cells: Key organisers in CNS development. Int. J. Biochem. Cell Biol. 2014, 46, 76–79. [Google Scholar] [CrossRef]
- Götz, M.; Nakafuku, M.; Petrik, D. Neurogenesis in the Developing and Adult Brain-Similarities and Key Differences. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Gauthier, A.S. Timing is everything: Making neurons versus glia in the developing cortex. Neuron 2007, 54, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Haydar, T.F.; Wang, F.; Schwartz, M.L.; Rakic, P. Differential Modulation of Proliferation in the Neocortical Ventricular and Subventricular Zones. J. Neurosci. 2000, 20, 5764–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamichi, N.; Takarada, T.; Yoneda, Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamate signaling. J. Pharmacol. Sci. 2009, 110, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Giachino, C.; Basak, O.; Lugert, S.; Knuckles, P.; Obernier, K.; Fiorelli, R.; Frank, S.; Raineteau, O.; Alvarez-Buylla, A.; Taylor, V. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells Dayt. Ohio 2014, 32, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Hylin, M.J.; Orsi, S.A.; Moore, A.N.; Dash, P.K. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn. Mem. Cold Spring Harb. N 2013, 20, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Kwok, J.C.F.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011, 71, 1073–1089. [Google Scholar] [CrossRef]
- Lemarchant, S.; Pomeshchik, Y.; Kidin, I.; Kärkkäinen, V.; Valonen, P.; Lehtonen, S.; Goldsteins, G.; Malm, T.; Kanninen, K.; Koistinaho, J. ADAMTS-4 promotes neurodegeneration in a mouse model of amyotrophic lateral sclerosis. Mol. Neurodegener. 2016, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Moon, L.D.; Asher, R.A.; Rhodes, K.E.; Fawcett, J.W. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci. 2001, 4, 465–466. [Google Scholar] [CrossRef]
- Isacson, O.; Dunnett, S.B.; Björklund, A. Graft-induced behavioral recovery in an animal model of Huntington disease. Proc. Natl. Acad. Sci. USA 1986, 83, 2728–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeck, J.A.; Studer, L. Moving stem cells to the clinic: Potential and limitations for brain repair. Neuron 2015, 86, 187–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadrato, G.; Elnaggar, M.Y.; Di Giovanni, S. Adult neurogenesis in brain repair: Cellular plasticity vs. cellular replacement. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheff, S.W.; Benardo, L.S.; Cotman, C.W. Hydrocortisone administration retards axon sprouting in the rat dentate gyrus. Exp. Neurol. 1980, 68, 195–201. [Google Scholar] [CrossRef]
- Rønn, L.C.B.; Berezin, V.; Bock, E. The neural cell adhesion molecule in synaptic plasticity and ageing. Int. J. Dev. Neurosci. 2000, 18, 193–199. [Google Scholar] [CrossRef]
- Hoffman, S.; Edelman, G.M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 1983, 80, 5762–5766. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, U.; Landmesser, L. Polysialic acid in the vertebrate nervous system: A promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996, 19, 422–427. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Brackenbury, R.; Cunningham, B.A.; Edelman, G.M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J. Biol. Chem. 1982, 257, 11064–11069. [Google Scholar]
- Di Cristo, G.; Chattopadhyaya, B.; Kuhlman, S.J.; Fu, Y.; Bélanger, M.-C.; Wu, C.Z.; Rutishauser, U.; Maffei, L.; Huang, Z.J. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 2007, 10, 1569–1577. [Google Scholar] [CrossRef]
- Jiang, Y.; Tong, D.; Hofacer, R.D.; Loepke, A.W.; Lian, Q.; Danzer, S.C. Long-term Fate Mapping to Assess the Impact of Postnatal Isoflurane Exposure on Hippocampal Progenitor Cell Productivity. Anesthesiology 2016, 125, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Battista, D.; Ganat, Y.; El Maarouf, A.; Studer, L.; Rutishauser, U. Enhancement of polysialic acid expression improves function of embryonic stem-derived dopamine neuron grafts in Parkinsonian mice. Stem Cells Transl. Med. 2014, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef] [PubMed]
- McKerracher, L.; David, S.; Jackson, D.L.; Kottis, V.; Dunn, R.J.; Braun, P.E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 1994, 13, 805–811. [Google Scholar] [CrossRef]
- Kottis, V.; Thibault, P.; Mikol, D.; Xiao, Z.-C.; Zhang, R.; Dergham, P.; Braun, P.E. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J. Neurochem. 2002, 82, 1566–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poplawski, G.H.D.; Lie, R.; Hunt, M.; Kumamaru, H.; Kawaguchi, R.; Lu, P.; Schäfer, M.K.E.; Woodruff, G.; Robinson, J.; Canete, P.; et al. Adult rat myelin enhances axonal outgrowth from neural stem cells. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, Y.; Stegmüller, J.; Matsuda, T.; Bonni, S.; Bonni, A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 2004, 303, 1026–1030. [Google Scholar] [CrossRef] [Green Version]
- Platel, J.-C.; Dave, K.A.; Gordon, V.; Lacar, B.; Rubio, M.E.; Bordey, A. NMDA Receptors Activated by Subventricular Zone Astrocytic Glutamate Are Critical for Neuroblast Survival Prior to Entering a Synaptic Network. Neuron 2010, 65, 859–872. [Google Scholar] [CrossRef] [Green Version]
- Whitney, N.P.; Eidem, T.M.; Peng, H.; Huang, Y.; Zheng, J.C. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J. Neurochem. 2009, 108, 1343–1359. [Google Scholar] [CrossRef]
- Ryan, S.M.; Nolan, Y.M. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neurosci. Biobehav. Rev. 2016, 61, 121–131. [Google Scholar] [CrossRef]
- Andsberg, G.; Björklund, Z.K.A.; Lindvall, O.; Martínez-Serrano, A. Amelioration of ischaemia-induced neuronal death in the rat striatum by NGF-secreting neural stem cells. Eur. J. Neurosci. 1998, 10, 2026–2036. [Google Scholar] [CrossRef]
- Chang, D.J.; Lee, N.; Choi, C.; Jeon, I.; Oh, S.H.; Shin, D.A.; Hwang, T.S.; Lee, H.J.; Kim, S.U.; Moon, H.; et al. Therapeutic effect of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) in a rodent model of middle cerebral artery occlusion. Cell Transplant. 2013, 22, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, R.; Wang, R.; Li, G.; Wei, J.; Li, Z.; Feng, M.; Kang, J.; Du, W.; Ma, W.; et al. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci. Lett. 2008, 444, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gao, X.-Q.; Yang, C.-X.; Tan, S.-K.; Sun, Z.-L.; Yan, N.-H.; Pang, Y.-G.; Yuan, M.; Chen, G.-J.; Xu, G.-T.; et al. Neuroprotective effect of grafting GDNF gene-modified neural stem cells on cerebral ischemia in rats. Brain Res. 2009, 1284, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Mao, Y.; Zhao, Y.; Zhou, L.-F.; Wang, Y.; Zhu, J.-H.; Zhu, Y.; Yang, G.-Y. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery 2005, 57, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Zhao, Y.Y.; Chen, S.D.; Zhang, W.H.; Lou, L.; Jin, X. Functional recovery after transplantation of neural stem cells modified by brain-derived neurotrophic factor in rats with cerebral ischaemia. J. Int. Med. Res. 2011, 39, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, G.M.; Avalos, P.; Ma, A.A.; Alkaslasi, M.; Cho, N.; Wyss, L.; Vit, J.-P.; Godoy, M.; Suezaki, P.; Shelest, O.; et al. Transplantation of Neural Progenitor Cells Expressing Glial Cell Line-Derived Neurotrophic Factor into the Motor Cortex as a Strategy to Treat Amyotrophic Lateral Sclerosis. Stem Cells Dayt. Ohio 2018, 36, 1122–1131. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Klein, S.M.; Aebischer, P.; Svendsen, C.N. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE 2007, 2, e689. [Google Scholar] [CrossRef]
- Behrstock, S.; Ebert, A.; McHugh, J.; Vosberg, S.; Moore, J.; Schneider, B.; Capowski, E.; Hei, D.; Kordower, J.; Aebischer, P.; et al. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006, 13, 379–388. [Google Scholar] [CrossRef]
- Khazaei, M.; Ahuja, C.S.; Nakashima, H.; Nagoshi, N.; Li, L.; Wang, J.; Chio, J.; Badner, A.; Seligman, D.; Ichise, A.; et al. GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lien, C.-C.; Hou, W.-H.; Chiang, P.-M.; Tsai, K.-J. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Gantner, C.W.; de Luzy, I.R.; Kauhausen, J.A.; Moriarty, N.; Niclis, J.C.; Bye, C.R.; Penna, V.; Hunt, C.P.J.; Ermine, C.M.; Pouton, C.W.; et al. Viral Delivery of GDNF Promotes Functional Integration of Human Stem Cell Grafts in Parkinson’s Disease. Cell Stem Cell 2020. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Long, L.; Tuo, Y.; Wang, L.; Zhao, X.; Le, W.; Wan, Y. Wnt4-modified NSC transplantation promotes functional recovery after spinal cord injury. FASEB J. 2020, 34, 82–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engle, S.J.; Blaha, L.; Kleiman, R.J. Best Practices for Translational Disease Modeling Using Human iPSC-Derived Neurons. Neuron 2018, 100, 783–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, S.; Stacey, G.N.; Akazawa, C.; Aoyama, N.; Baptista, R.; Bedford, P.; Bennaceur Griscelli, A.; Chandra, A.; Elwood, N.; Girard, M.; et al. Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen. Med. 2018, 13, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Marker | References | |
|---|---|---|
| Neuronal stem cell | Paired box 6 (PAX6) | [20,21,22] |
| SRY-box transcription factor 1 (SOX1) | [21,22,23,24] | |
| SRY-box transcription factor 2 (SOX2) | [21,22,23,24] | |
| Nestin (NES) | [21,25] | |
| Cut-like homeobox 1/2 (CUX1/2) | [22,26] | |
| Notch homolog 1 (Notch1) | [26,27] | |
| Hairy and enhancer of split 1/3/5 (HES1/3/5) | [22,23,26,27] | |
| Cadherin-1/2 (CDH1/2) | [26] | |
| SRY-box transcription factor 10 (SOX10) | [26] | |
| Vimentin (VIM) | [22,25] | |
| Glial fibrillary acidic protein (GFAP) | [22,25] | |
| Neuronal precursors | Microtubule-associated protein 2 (MAP2) | [28,29] |
| Class III β-tubulin (TuJ1) | [22] | |
| Doublecortin (DCX) | [22,30] | |
| ELAV-like protein 3/4 (HuC/D) | [31] | |
| Neurofilament (NF) | [21,25] |
| Marker | References | |
|---|---|---|
| Pluripotent cells | Stage-specific embryonic antigen 3 (SSEA-3) | [15,32] |
| Stage-specific embryonic antigen 4 (SSEA-4) | ||
| T cell receptor α locus 1-60 (TRA-1-60) | ||
| T cell receptor α locus 1-81 (TRA-1-81) | ||
| Early Neural Commitment | Prominin-1 (CD133) | [32,33] |
| Lewis X antigen (CD15) | ||
| Forebrain-surface-embryonic antigen-1 (FORSE-1) | ||
| Melanoma cell adhesion molecule (CD146) | [16,32] | |
| P75 neurotrophin receptor (p75) | ||
| CXCR4, C-X-C chemokine receptor type 4 (CD184) | [28,32] | |
| Integrin β-1 (CD29) | [32] | |
| Integrin α-4 (CD49d) | ||
| Neural cell adhesion molecule L1 (CD171) | ||
| Epithelial cell adhesion molecule (CD326) | [34] | |
| Differentiated Neuronal Cells | Neural cell adhesion molecule (CD56) | [16,17] |
| Heat stable antigen (CD24) | ||
| Glial Precursor Cells | Neuron cell surface antigen A2B5 (A2B5) | [16] |
| Astrocyte Precursor Cells | Homing cell adhesion molecule (CD44) | [28] |
| Oligodendrocyte Precursor Cells | Platelet-derived growth factor receptor-α (CD140a) | [35] |
| Combinatorial Antigens | Cell Phenotype | Reference |
|---|---|---|
| CD15(+)/CD29(HI)/CD24(LO) | Neural Stem Cells | [17] |
| CD15(-)/CD29(HI)/CD24(LO) | Mesenchymal Stem Cells | |
| CD15(-)/CD29(LO)/CD24(HI) | Neuroblasts and Neurons | |
| CD184+/CD271–/CD44–/CD24+ | Neural Stem Cells | [28] |
| CD184–/CD44–/CD15 (LO)/CD24+ | Mature Neurons | |
| CD49f–/CD200(HI) | Neural Cells | [33] |
| Growth Factor | Reference |
|---|---|
| Brain-derived neurotrophic factor (BDNF) | [11,12,45,47] |
| Vascular endothelial growth factor (VEGF) | [11,49] |
| Glial-cell-line-derived neurotrophic factor (GDNF) | [12] |
| Nerve growth factor (NGF) | [11,45,46] |
| Neurotrophin-3 (NT3) | [11,12,45] |
| Basic fibroblast growth factor (BFGF) | [49] |
| Epidermal growth factor (EGF) | [49] |
| Insulin-like growth factor-1 (IGF-1) | [50] |
| Insulin-like growth factor-2 (IGF-2) | [51] |
| Ciliary neurotrophic factor (CNTF) | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gioia, R.; Biella, F.; Citterio, G.; Rizzo, F.; Abati, E.; Nizzardo, M.; Bresolin, N.; Comi, G.P.; Corti, S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3103. https://doi.org/10.3390/ijms21093103
De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, Bresolin N, Comi GP, Corti S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. International Journal of Molecular Sciences. 2020; 21(9):3103. https://doi.org/10.3390/ijms21093103
Chicago/Turabian StyleDe Gioia, Roberta, Fabio Biella, Gaia Citterio, Federica Rizzo, Elena Abati, Monica Nizzardo, Nereo Bresolin, Giacomo Pietro Comi, and Stefania Corti. 2020. "Neural Stem Cell Transplantation for Neurodegenerative Diseases" International Journal of Molecular Sciences 21, no. 9: 3103. https://doi.org/10.3390/ijms21093103