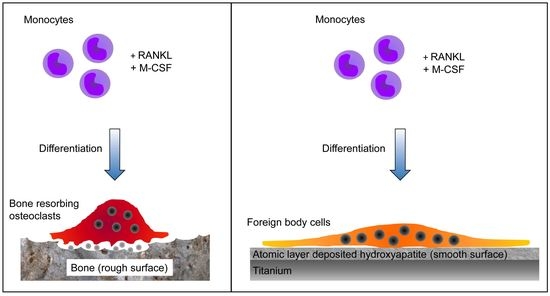
Monocyte Differentiation on Atomic Layer-Deposited (ALD) Hydroxyapatite Coating on Titanium Substrate
Abstract
:1. Introduction
2. Results
2.1. Contact Angle and Surface Roughness Measurements
2.2. Multinuclear Foreign Body Cells Generated on ALD-HA
3. Discussion
4. Materials and Methods
4.1. Preparation of Nanocrystalline HA Coating (ALD-HA) on Ti Substrates with ALD Method
4.2. Contact Angle and Surface Roughness Measurements
4.3. Monocyte Differentiation on Bone and ALD-HA
4.4. Confocal Microscopy
4.5. Field Emission Scanning Electron Microscopy (FESEM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Balamurugan, A.; Rajeswari, S.; Balossier, G.; Rebelo, A.H.S.; Ferreira, J.M.F. Corrosion Aspects of Metallic Implants—An Overview. Mater. Corros. 2008, 59, 855–869. [Google Scholar] [CrossRef]
- Cheng, C.K.; Wang, X.H.; Luan, Y.C.; Zhang, N.Z.; Liu, B.L.; Ma, X.Y.; Nie, M.D. Challenges of Pre-Clinical Testing in Orthopedic Implant Development. Med. Eng. Phys. 2019, 72, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated Titanium Implants. Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, D.T.P.; Berry, D.J. The Epidemiology of Revision Total Hip Arthroplasty in the United States. J. Bone Jt. Surg. 2009, 91, 128–133. [Google Scholar] [CrossRef]
- Nobles, K.P.; Janorkar, A.V.; Williamson, R.S. Surface Modifications to Enhance Osseointegration–Resulting Material Properties and Biological Responses. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1909–1923. [Google Scholar] [CrossRef]
- Schroer, W.C.; Berend, K.R.; Lombardi, A.V.; Barnes, C.L.; Bolognesi, M.P.; Berend, M.E.; Ritter, M.A.; Nunley, R.M. Why Are Total Knees Failing Today? Etiology of Total Knee Revision in 2010 and 2011. J. Arthroplast. 2013, 28, 116–119. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface Modification of Titanium, Titanium Alloys, and Related Materials for Biomedical Applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Hao, J.; Kuroda, S.; Ohya, K.; Bartakova, S.; Aoki, H.; Kasugai, S. Enhanced Osteoblast and Osteoclast Responses to a Thin Film Sputtered Hydroxyapatite Coating. J. Mater. Sci. Mater. Med. 2011, 22, 1489–1499. [Google Scholar] [CrossRef]
- Landor, I.; Vavrik, P.; Sosna, A.; Jahoda, D.; Hahn, H.; Daniel, M. Hydroxyapatite Porous Coating and the Osteointegration of the Total Hip Replacement. Arch. Orthop. Trauma Surg. 2007, 127, 81–89. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.P.; Spector, M.; Hobbs, L.W. Early Bone Apposition in Vivo on Plasma-Sprayed and Electrochemically Deposited Hydroxyapatite Coatings on Titanium Alloy. Biomaterials 2006, 27, 4192–4203. [Google Scholar] [CrossRef]
- Coathup, M.J.; Blunn, G.W.; Flynn, N.; Williams, C.; Thomas, N.P. A Comparison of Bone Remodelling around Hydroxyapatite-Coated, Porous-Coated and Grit-Blasted Hip Replacements Retrieved at Post-Mortem. J. Bone Jt. Surg. Br. 2001, 83, 118–123. [Google Scholar] [CrossRef]
- Daugaard, H.; Elmengaard, B.; Bechtold, J.E.; Jensen, T.; Soballe, K. The Effect on Bone Growth Enhancement of Implant Coatings with Hydroxyapatite and Collagen Deposited Electrochemically and by Plasma Spray. J. Biomed. Mater. Res. A 2010, 92, 913–921. [Google Scholar] [CrossRef]
- Porter, A.E.; Hobbs, L.W.; Rosen, V.B.; Spector, M. The Ultrastructure of the Plasma-Sprayed Hydroxyapatite–Bone Interface Predisposing to Bone Bonding. Biomaterials 2002, 23, 725–733. [Google Scholar] [CrossRef]
- Aebli, N.; Krebs, J.; Schwenke, D.; Stich, H.; Schwalder, P.; Theis, J.C. Degradation of Hydroxyapatite Coating on a Well-Functioning Femoral Component. J. Bone Jt. Surg. Ser. B 2003, 85, 499–503. [Google Scholar] [CrossRef]
- Overgaard, S.; Lind, M.; Josephsen, K.; Maunsbach, A.B.; Bünger, C.; Søballe, K. Resorption of Hydroxyapatite and Fluorapatite Ceramic Coatings on Weight-Bearing Implants: A Quantitative and Morphological Study in Dogs. J. Biomed. Mater. Res. 1998, 39, 141–152. [Google Scholar] [CrossRef]
- Tonino, A.J.; Thèrin, M.; Doyle, C. Hydroxyapatite-Coated Femoral Stems. Histology and Histomorphometry around Five Components Retrieved at Post Mortem. J. Bone Jt. Surg. Br. 1999, 81, 148–154. [Google Scholar] [CrossRef]
- Tonino, A.; van der Wal, B.; Heyligers, I.; Grimm, B. Bone Remodeling and Hydroxyapatite Resorption in Coated Primary Hip Prostheses. Clin. Orthop. Relat. Res. 2009, 467, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone Remodeling: An Operational Process Ensuring Survival and Bone Mechanical Competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Lee, J.W.; Ju, C.P.; Lin, J.H.C. Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material. Materials 2020, 13, 3458. [Google Scholar] [CrossRef] [PubMed]
- Haga, M.; Fujii, N.; Nozawa-Inoue, K.; Nomura, S.; Oda, K.; Uoshima, K.; Maeda, T. Detailed Process of Bone Remodeling after Achievement of Osseointegration in a Rat Implantation Model. Anat. Rec. 2009, 292, 38–47. [Google Scholar] [CrossRef]
- Wenisch, S.; Stahl, J.-P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In Vivo Mechanisms of Hydroxyapatite Ceramic Degradation by Osteoclasts: Fine Structural Microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef]
- Akiyama, N.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Hirano, M.; Nakamura, T. Difference between Dogs and Rats with Regard to Osteoclast-like Cells in Calcium-Deficient Hydroxyapatite-Induced Osteoinduction. J. Biomed. Mater. Res. A 2011, 96A, 402–412. [Google Scholar] [CrossRef]
- Kondo, N.; Ogose, A.; Tokunaga, K.; Umezu, H.; Arai, K.; Kudo, N.; Hoshino, M.; Inoue, H.; Irie, H.; Kuroda, K.; et al. Osteoinduction with Highly Purified β-Tricalcium Phosphate in Dog Dorsal Muscles and the Proliferation of Osteoclasts before Heterotopic Bone Formation. Biomaterials 2006, 27, 4419–4427. [Google Scholar] [CrossRef]
- Nasu, T.; Takemoto, M.; Akiyama, N.; Fujibayashi, S.; Neo, M.; Nakamura, T. EP4 Agonist Accelerates Osteoinduction and Degradation of P-Tricalcium Phosphate by Stimulating Osteoclastogenesis. J. Biomed. Mater. Res. A 2009, 89, 601–608. [Google Scholar] [CrossRef]
- Davison, N.L.; Gamblin, A.L.; Layrolle, P.; Yuan, H.; de Bruijn, J.D.; Barrère-de Groot, F. Liposomal Clodronate Inhibition of Osteoclastogenesis and Osteoinduction by Submicrostructured Beta-Tricalcium Phosphate. Biomaterials 2014, 35, 5088–5097. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Ravikumar, K.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Ahmadzadeh, K.; Vanoppen, M.; Rose, C.D.; Matthys, P.; Wouters, C.H. Multinucleated Giant Cells: Current Insights in Phenotype, Biological Activities, and Mechanism of Formation. Front. Cell Dev. Biol. 2022, 10, 873226. [Google Scholar] [CrossRef]
- ten Harkel, B.; Schoenmaker, T.; Picavet, D.I.; Davison, N.L.; de Vries, T.J.; Everts, V. The Foreign Body Giant Cell Cannot Resorb Bone, But Dissolves Hydroxyapatite Like Osteoclasts. PLoS ONE 2015, 10, e0139564. [Google Scholar] [CrossRef]
- Khan, U.A.; Hashimi, S.M.; Bakr, M.M.; Forwood, M.R.; Morrison, N.A. Foreign Body Giant Cells and Osteoclasts Are TRAP Positive, Have Podosome-Belts and Both Require OC-STAMP for Cell Fusion. J. Cell. Biochem. 2013, 114, 1772–1778. [Google Scholar] [CrossRef]
- Miron, R.J.; Zohdi, H.; Fujioka-Kobayashi, M.; Bosshardt, D.D. Giant Cells around Bone Biomaterials: Osteoclasts or Multi-Nucleated Giant Cells? Acta Biomater. 2016, 46, 15–28. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Machado, M.; Jasty, M.; Jevsevar, D.; Wolfe, H.J.; Goldring, S.R.; Goldberg, M.J.; Harris, W.H. Production of Cytokines around Loosened Cemented Acetabular Components. Analysis with Immunohistochemical Techniques and in Situ Hybridization. J. Bone Jt. Surg. Am. 1993, 75, 863–879. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Bornstein, Q.L.; Aguilar Leon, D.; Orozco, E.H.; Madrigal, V.K.; Martinez Cordero, E. Inflammatory Cytokine Production by Immunological and Foreign Body Multinucleated Giant Cells. Immunology 2000, 100, 352. [Google Scholar] [CrossRef]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Il, K.K.; Matsuda, T.; Anderson, J.M. Proteomic Analysis and Quantification of Cytokines and Chemokines from Biomaterial Surface-Adherent Macrophages and Foreign Body Giant Cells. J. Biomed. Mater. Res. A 2007, 83, 585–596. [Google Scholar] [CrossRef]
- Sabokbar, A.; Pandey, R.; Quinn, J.M.; Athanasou, N.A. Osteoclastic Differentiation by Mononuclear Phagocytes Containing Biomaterial Particles. Arch. Orthop. Trauma Surg. 1998, 117, 136–140. [Google Scholar] [CrossRef]
- Pandey, R.; Quinn, J.; Joyner, C.; Murray, D.W.; Triffitt, J.T.; Athanasou, N.A. Arthroplasty Implant Biomaterial Particle Associated Macrophages Differentiate into Lacunar Bone Resorbing Cells. Ann. Rheum. Dis. 1996, 55, 388–395. [Google Scholar] [CrossRef]
- Morishita, K.; Tatsukawa, E.; Shibata, Y.; Suehiro, F.; Kamitakahara, M.; Yokoi, T.; Ioku, K.; Umeda, M.; Nishimura, M.; Ikeda, T. Diversity of Multinucleated Giant Cells by Microstructures of Hydroxyapatite and Plasma Components in Extraskeletal Implantation Model. Acta Biomater. 2016, 39, 180–191. [Google Scholar] [CrossRef]
- de Freitas Costa, N.; Melo, B.R.; Brito, R.T.; de Oliveira Fernandes, M.B.; Bernardo, V.G.; Fonseca, E.C.; Conz, M.B.; Soares, G.A.; Granjeiro, J.M. Quality and Intensity of the Tissue Response to Two Synthetic Granular Hydroxyapatite Implanted in Critical Defects of Rat Calvaria. Mater. Res. 2009, 12, 245–251. [Google Scholar] [CrossRef]
- Abels, M.; Alkildani, S.; Pröhl, A.; Xiong, X.; Krastev, R.; Korzinskas, T.; Stojanovic, S.; Jung, O.; Najman, S.; Barbeck, M. The Granule Size Mediates the In Vivo Foreign Body Response and the Integration Behavior of Bone Substitutes. Materials 2021, 14, 7372. [Google Scholar] [CrossRef] [PubMed]
- Herde, K.; Hartmann, S.; Brehm, R.; Kilian, O.; Heiss, C.; Hild, A.; Alt, V.; Bergmann, M.; Schnettler, R.; Wenisch, S. Connexin 43 Expression of Foreign Body Giant Cells after Implantation of Nanoparticulate Hydroxyapatite. Biomaterials 2007, 28, 4912–4921. [Google Scholar] [CrossRef] [PubMed]
- Sabokbar, A.; Pandey, R.; Díaz, J.; Quinn, J.M.; Murray, D.W.; Athanasou, N.A. Hydroxyapatite Particles Are Capable of Inducing Osteoclast Formation. J. Mater. Sci. Mater. Med. 2001, 12, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Monchau, F.; Lefèvre, A.; Descamps, M.; Belquin-myrdycz, A.; Laffargue, P.; Hildebrand, H.F. In Vitro Studies of Human and Rat Osteoclast Activity on Hydroxyapatite, β-Tricalcium Phosphate, Calcium Carbonate. Biomol. Eng. 2002, 19, 143–152. [Google Scholar] [CrossRef]
- Nakamura, M.; Hentunen, T.; Salonen, J.; Nagai, A.; Yamashita, K. Characterization of Bone Mineral-Resembling Biomaterials for Optimizing Human Osteoclast Differentiation and Resorption. J. Biomed. Mater. Res. A 2013, 101, 3141–3151. [Google Scholar] [CrossRef]
- Yamada, S.; Heymann, D.; Bouler, J.M.; Daculsi, G. Osteoclastic Resorption of Calcium Phosphate Ceramics with Different Hydroxyapatite/β-Tricalcium Phosphate Ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef]
- Doi, Y.; Iwanaga, H.; Shibutani, T.; Moriwaki, Y.; Iwayama, Y. Osteoclastic Responses to Various Calcium Phosphates in Cell Cultures. J. Biomed. Mater. Res. 1999, 47, 424–433. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Wang, J.; Chen, X.; Li, X.; Xiao, Y.; Zhang, X. Effects of Hydroxyapatite Surface Nano/Micro-Structure on Osteoclast Formation and Activity. J. Mater. Chem. B 2019, 7, 7574–7587. [Google Scholar] [CrossRef]
- Davison, N.L.; Su, J.; Yuan, H.; van den Beucken, J.J.J.P.; de Bruijn, J.D.; de Groot, F.B. Influence of Surface Microstructure and Chemistry on Osteoinduction and Osteoclastogenesis by Biphasic Calcium Phosphate Discs. Eur. Cell Mater. 2015, 29, 314–329. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, A.; Lopes, M.A.; Fernandes, M.H. Hydroxyapatite Surface Roughness: Complex Modulation of the Osteoclastogenesis of Human Precursor Cells. Acta Biomater. 2012, 8, 1137–1145. [Google Scholar] [CrossRef]
- Costa, D.O.; Prowse, P.D.H.; Chrones, T.; Sims, S.M.; Hamilton, D.W.; Rizkalla, A.S.; Dixon, S.J. The Differential Regulation of Osteoblast and Osteoclast Activity by Surface Topography of Hydroxyapatite Coatings. Biomaterials 2013, 34, 7215–7226. [Google Scholar] [CrossRef]
- Gross, K.A.; Muller, D.; Lucas, H.; Haynes, D.R. Osteoclast Resorption of Thermal Spray Hydoxyapatite Coatings Is Influenced by Surface Topography. Acta Biomater. 2012, 8, 1948–1956. [Google Scholar] [CrossRef]
- Ciapetti, G.; di Pompo, G.; Avnet, S.; Martini, D.; Diez-Escudero, A.; Montufar, E.B.; Ginebra, M.P.; Baldini, N. Osteoclast Differentiation from Human Blood Precursors on Biomimetic Calcium-Phosphate Substrates. Acta Biomater. 2017, 50, 102–113. [Google Scholar] [CrossRef]
- He, Y.; Gao, Y.; Ma, Q.; Zhang, X.; Zhang, Y.; Song, W. Nanotopographical Cues for Regulation of Macrophages and Osteoclasts: Emerging Opportunities for Osseointegration. J. Nanobiotechnol. 2022, 20, 510. [Google Scholar] [CrossRef]
- Shiwaku, Y.; Neff, L.; Nagano, K.; Takeyama, K.I.; de Bruijn, J.; Dard, M.; Gori, F.; Baron, R. The Crosstalk between Osteoclasts and Osteoblasts Is Dependent upon the Composition and Structure of Biphasic Calcium Phosphates. PLoS ONE 2015, 10, e0132903. [Google Scholar] [CrossRef]
- Thevenot, P.; Hu, W.; Tang, L. Surface chemistry influence implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of Macrophage Polarization through Surface Topography Design to Facilitate Implant-to-Bone Osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.Y.; Anderson, J.M.; Leong, K.W. Characterization of Topographical Effects on Macrophage Behavior in a Foreign Body Response Model. Biomaterials 2010, 31, 3479. [Google Scholar] [CrossRef]
- Robotti, F.; Bottan, S.; Fraschetti, F.; Mallone, A.; Pellegrini, G.; Lindenblatt, N.; Starck, C.; Falk, V.; Poulikakos, D.; Ferrari, A. A Micron-Scale Surface Topography Design Reducing Cell Adhesion to Implanted Materials. Sci. Rep. 2018, 8, 10887. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite Coatings for Metallic Implants. In Hydroxyapatite (Hap) for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 143–157. [Google Scholar]
- Faig-Martí, J.; Gil-Mur, F.J. Hydroxyapatite Coatings in Prosthetic Joints. Rev. Española Cirugía Ortopédica Traumatol. (Engl. Ed.) 2008, 52, 113–120. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material Fundamentals and Clinical Performance of Plasma-Sprayed Hydroxyapatite Coatings: A Review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Astaneh, S.; Faverani, L.P.; Sukotjo, C.; Takoudis, C.G. Atomic Layer Deposition on Dental Materials: Processing Conditions and Surface Functionalization to Improve Physical, Chemical, and Clinical Properties—A Review. Acta Biomater. 2021, 121, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New Development of Atomic Layer Deposition: Processes, Methods and Applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Holopainen, J.; Kauppinen, K.; Mizohata, K.; Santala, E.; Mikkola, E.; Heikkilä, M.; Kokkonen, H.; Leskelä, M.; Lehenkari, P.; Tuukkanen, J.; et al. Preparation and Bioactive Properties of Nanocrystalline Hydroxyapatite Thin Films Obtained by Conversion of Atomic Layer Deposited Calcium Carbonate. Biointerphases 2014, 9, 031008. [Google Scholar] [CrossRef]
- Avila, I.; Pantchev, K.; Holopainen, J.; Ritala, M.; Tuukkanen, J. Adhesion and Mechanical Properties of Nanocrystalline Hydroxyapatite Coating Obtained by Conversion of Atomic Layer-Deposited Calcium Carbonate on Titanium Substrate. J. Mater. Sci. Mater. Med. 2018, 29, 111. [Google Scholar] [CrossRef]
- Kylmäoja, E.; Holopainen, J.; Abushahba, F.; Ritala, M.; Tuukkanen, J. Osteoblast Attachment on Titanium Coated with Hydroxyapatite by Atomic Layer Deposition. Biomolecules 2022, 12, 654. [Google Scholar] [CrossRef]
- Kylmäoja, E.; Nakamura, M.; Turunen, S.; Patlaka, C.; Andersson, G.; Lehenkari, P.; Tuukkanen, J. Peripheral Blood Monocytes Show Increased Osteoclast Differentiation Potential Compared to Bone Marrow Monocytes. Heliyon 2018, 4, e00780. [Google Scholar] [CrossRef]
- Chappard, D.; Kün-Darbois, J.D.; Pascaretti-Grizon, F.; Camprasse, G.; Camprasse, S. Giant Cells and Osteoclasts Present in Bone Grafted with Nacre Differ by Nuclear Cytometry Evaluated by Texture Analysis. J. Mater. Sci. Mater. Med. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Redey, S.A.; Razzouk, S.; Rey, C.; Bernache-Assollant, D.; Leroy, G. Osteoclast Adhesion and Activity on Synthetic Hydroxyapatite, Carbonated Hydroxyapatite, and Natural Calcium Carbonate: Relationship to Surface Energies. J. Biomed. Mater. Res. 1999, 45, 140–147. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; James Kirkpatrick, C.; Ghanaati, S. In Vivo Cellular Reactions to Different Biomaterials—Physiological and Pathological Aspects and Their Consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Ghanaati, S.; Udeabor, S.E.; Barbeck, M.; Willershausen, I.; Kuenzel, O.; Sader, R.A.; Kirkpatrick, J. Implantation of Silicon Dioxide-Based Nanocrystalline Hydroxyapatite and Pure Phase Beta-Tricalciumphosphate Bone Substitute Granules in Caprine Muscle Tissue Does Not Induce New Bone Formation. Head Face Med. 2013, 9, 1. [Google Scholar] [CrossRef]
- Okuda, T.; Ioku, K.; Yonezawa, I.; Minagi, H.; Gonda, Y.; Kawachi, G.; Kamitakahara, M.; Shibata, Y.; Murayama, H.; Kurosawa, H.; et al. The Slow Resorption with Replacement by Bone of a Hydrothermally Synthesized Pure Calcium-Deficient Hydroxyapatite. Biomaterials 2008, 29, 2719–2728. [Google Scholar] [CrossRef]
- Brinkmann, J.; Hefti, T.; Schlottig, F.; Spencer, N.D.; Hall, H. Response of Osteoclasts to Titanium Surfaces with Increasing Surface Roughness: An in Vitro Study. Biointerphases 2012, 7, 34. [Google Scholar] [CrossRef]
- Makihira, S.; Mine, Y.; Kosaka, E.; Nikawa, H. Titanium Surface Roughness Accelerates RANKL-Dependent Differentiation in the Osteoclast Precursor Cell Line, RAW264.7. Dent. Mater. J. 2007, 26, 739–745. [Google Scholar] [CrossRef]
- Sommer, B.; Felix, R.; Sprecher, C.; Leunig, M.; Ganz, R.; Hofstetter, W. Wear Particles and Surface Topographies Are Modulators of Osteoclastogenesis in Vitro. J. Biomed. Mater. Res. A 2005, 72, 67–76. [Google Scholar] [CrossRef]
- Nagasawa, M.; Cooper, L.F.; Ogino, Y.; Mendonca, D.; Liang, R.; Yang, S.; Mendonca, G.; Uoshima, K. Topography Influences Adherent Cell Regulation of Osteoclastogenesis. J. Dent. Res. 2016, 95, 319–326. [Google Scholar] [CrossRef]
- Roy, M.; Bose, S. Osteoclastogenesis and Osteoclastic Resorption of Tricalcium Phosphate: Effect of Strontium and Magnesium Doping. J. Biomed. Mater. Res. A 2012, 100A, 2450–2461. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Kleinhans, C.; Vacun, G.; Kluger, P.J.; Schönhaar, V.; Müller, M.; Hein, S.B.; Wittmar, A.; Ulbricht, M.; Prymak, O.; et al. Nano-Hydroxyapatite-Coated Metal-Ceramic Composite of Iron-Tricalcium Phosphate: Improving the Surface Wettability, Adhesion and Proliferation of Mesenchymal Stem Cells in Vitro. Colloids Surf. B Biointerfaces 2015, 135, 386–393. [Google Scholar] [CrossRef]
- Davison, N.L.; Luo, X.; Schoenmaker, T.; Everts, V.; Yuan, H.; Barrère-de Groot, F.; de Bruijn, J.D. Submicron-Scale Surface Architecture of Tricalcium Phosphate Directs Osteogenesis in Vitro and in Vivo. Eur. Cell Mater. 2014, 27, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Davison, N.L.; ten Harkel, B.; Schoenmaker, T.; Luo, X.; Yuan, H.; Everts, V.; Barrère-de Groot, F.; de Bruijn, J.D. Osteoclast Resorption of Beta-Tricalcium Phosphate Controlled by Surface Architecture. Biomaterials 2014, 35, 7441–7451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.C.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. The Size of Surface Microstructures as an Osteogenic Factor in Calcium Phosphate Ceramics. Acta Biomater. 2014, 10, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Carmo, S.; Perpétuo, I.P.; Monteiro, F.J.; Fernandes, M.H. Osteoclastogenic Differentiation of Human Precursor Cells over Micro- and Nanostructured Hydroxyapatite Topography. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 825–835. [Google Scholar] [CrossRef]
- Mohammed Mohammed, A.H.; Shariff, K.A.; Bakar, M.H.A.; Mohamad, H. A Review on the Behavioral Responses of Osteoclast and Osteoblast Cells on the Near-Surface of the Bioceramic Coating: Roles of Ions Released, Solubility, and PH. J. Aust. Ceram. Soc. 2022, 58, 1715–1727. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Smieszek, A.; Seweryn, A.; Marcinkowska, K.; Sikora, M.; Lawniczak-Jablonska, K.; Witkowski, B.S.; Kuzmiuk, P.; Godlewski, M.; Marycz, K. Titanium Dioxide Thin Films Obtained by Atomic Layer Deposition Promotes Osteoblasts’ Viability and Differentiation Potential While Inhibiting Osteoclast Activity—Potential Application for Osteoporotic Bone Regeneration. Materials 2020, 13, 4817. [Google Scholar] [CrossRef]
- Zvaifler, N.J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C.J.; Moss, J.; Burger, J.A.; Maini, R.N. Mesenchymal Precursor Cells in the Blood of Normal Individuals. Arthritis Res. 2000, 2, 477–488. [Google Scholar] [CrossRef]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of Mesenchymal Stem Cells from G-CSF-Mobilized Human Peripheral Blood Using Fibrin Microbeads. Bone Marrow Transplant. 2006, 37, 967–976. [Google Scholar] [CrossRef]
- Karst, M.; Gorny, G.; Sells Galvin, R.J.; Oursler, M.J. Roles of Stromal Cell RANKL, OPG, and M-CSF Expression in Biphasic TGF-b Regulation of Osteoclast Differentiation. J. Cell. Physiol. 2004, 200, 99–106. [Google Scholar] [CrossRef]
- Sharaf-Eldin, W.E.; Abu-Shahba, N.; Mahmoud, M.; El-Badri, N. The Modulatory Effects of Mesenchymal Stem Cells on Osteoclastogenesis. Stem Cells Int. 2016, 2016, 1908365. [Google Scholar] [CrossRef]
- Nilsen, O.; Fjellvåg, H.; Kjekshus, A. Growth of Calcium Carbonate by the Atomic Layer Chemical Vapour Deposition Technique. Thin Solid Film. 2004, 450, 240–247. [Google Scholar] [CrossRef]
- de Jong, H.P.; van Pelt, A.W.J.; Arends, J. Contact Angle Measurements on Human Enamel—An in Vitro Study of Influence of Pellicle and Storage Period. J. Dent. Res. 1982, 61, 11–13. [Google Scholar] [CrossRef]
- Susa, M.; Luong-Nguyen, N.H.; Cappellen, D.; Zamurovic, N.; Gamse, R. Human Primary Osteoclasts: In Vitro Generation and Applications as Pharmacological and Clinical Assay. J. Transl. Med. 2004, 2, 6. [Google Scholar] [CrossRef]



| Contact Angle (°), Mean (SD) | Surface Roughness (Ra; µm), Mean (SD) | |
|---|---|---|
| Non-coated Ti sample | 86.9 (3.2) | 1.21 a (0.05) |
| ALD-HA | 86.2 (4.5) | 0.713 b (0.08) |
| Bone | 86.7 (4.5) | 2.30 (0.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kylmäoja, E.; Abushahba, F.; Holopainen, J.; Ritala, M.; Tuukkanen, J. Monocyte Differentiation on Atomic Layer-Deposited (ALD) Hydroxyapatite Coating on Titanium Substrate. Molecules 2023, 28, 3611. https://doi.org/10.3390/molecules28083611
Kylmäoja E, Abushahba F, Holopainen J, Ritala M, Tuukkanen J. Monocyte Differentiation on Atomic Layer-Deposited (ALD) Hydroxyapatite Coating on Titanium Substrate. Molecules. 2023; 28(8):3611. https://doi.org/10.3390/molecules28083611
Chicago/Turabian StyleKylmäoja, Elina, Faleh Abushahba, Jani Holopainen, Mikko Ritala, and Juha Tuukkanen. 2023. "Monocyte Differentiation on Atomic Layer-Deposited (ALD) Hydroxyapatite Coating on Titanium Substrate" Molecules 28, no. 8: 3611. https://doi.org/10.3390/molecules28083611