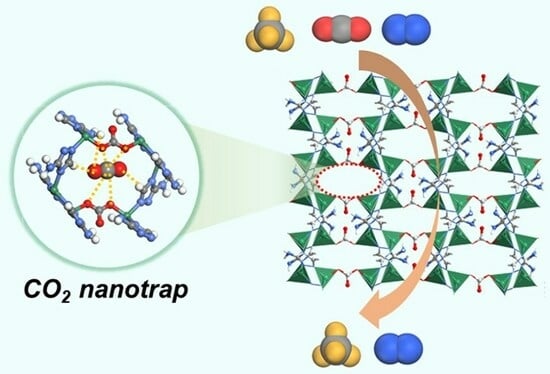
Efficient Selective Capture of Carbon Dioxide from Nitrogen and Methane Using a Metal-Organic Framework-Based Nanotrap
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure and Pore Properties
2.2. Characterizations
2.3. Adsorption Equilibrium Behavior of CO2, N2, and CH4
2.4. Molecular Simulations on the Selective CO2 Adsorption over N2 and CH4
2.5. Dynamic Breakthrough Experiments
3. Materials and Methods
3.1. Material Sources
3.2. Synthesis of ZnAtzCO3
3.3. Characterizations
3.4. Single-Component Gas Sorption Isotherm Measurements
3.5. Adsorption Selectivity Based on IAST Model
3.6. Isosteric Heat (Qst) Calculation
3.7. Breakthrough Experiments
3.8. Simulation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourzac, K. We have the technology. Nature 2017, 550, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Schuur, E.A.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Rogelj, J.; Séférian, R.; Wartenburger, R.; Allen, M.R.; Cain, M.; Millar, R.J.; Ebi, K.L.; Ellis, N.; Hoegh-Guldberg, O. The many possible climates from the Paris agreement’s aim of 1.5 C warming. Nature 2018, 558, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Iftikhar, T.; Ahmad, K.; Qureshi, K.; Tabinda; Altaf, F.; Iqbal, A.; Ahmad, S.; Khalid, A. Bulk industrial production of sustainable cellulosic printing fabric using agricultural waste to reduce the impact of climate change. Int. J. Biol. Macromol. 2023, 253, 126885. [Google Scholar] [CrossRef]
- Trends in Atmospheric Carbon Dioxide. Available online: https://gml.noaa.gov/ccgg/trends/mlo.html (accessed on 10 February 2021).
- International Energy Agency. CO2 Emissions in 2022; International Energy Agency: Paris, France, 2023.
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Assunção, L.R.; Mendes, P.A.; Matos, S.; Borschiver, S. Technology roadmap of renewable natural gas: Identifying trends for research and development to improve biogas upgrading technology management. Appl. Energy 2021, 292, 116849. [Google Scholar] [CrossRef]
- Ning, H.; Li, Y.; Zhang, C. Recent progress in the integration of CO2 capture and utilization. Molecules 2023, 28, 4500. [Google Scholar] [CrossRef]
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced strategies in metal-organic frameworks for CO2 capture and separation. Chem. Rec. 2022, 22, e202100230. [Google Scholar] [CrossRef]
- Modak, A.; Bhaumik, A. Porous carbon derived via KOH activation of a hypercrosslinked porous organic polymer for efficient CO2, CH4, H2 adsorptions and high CO2/N2 selectivity. J. Solid State Chem. 2015, 232, 157–162. [Google Scholar] [CrossRef]
- Kundu, S.K.; Bhaumik, A. Novel nitrogen and sulfur rich hyper-cross-linked microporous poly-triazine-thiophene copolymer for superior CO2 capture. ACS Sustain. Chem. Eng. 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Gao, X.; Yan, W.-H.; Hu, B.-Y.; Huang, Y.-X.; Zheng, S.-M. Porous metal-organic frameworks for light hydrocarbon separation. Molecules 2023, 28, 6337. [Google Scholar] [CrossRef] [PubMed]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Rajendran, A.; Subraveti, S.G.; Pai, K.N.; Prasad, V.; Li, Z. How can (or why should) process engineering aid the screening and discovery of solid sorbents for CO2 capture? Acc. Chem. Res. 2023, 56, 2354–2365. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, P.; Tong, Z.; Zhao, Z.; Zhao, Z. Enhanced hydrophobic MIL(Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air. Chem. Eng. J. 2017, 313, 1122–1131. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A.; et al. Engineering of zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Z.-Z.; Xiang, S.; Chen, B. Perspective of microporous metal-organic frameworks for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Kim, Y.; Huh, S. Pore engineering of metal-organic frameworks: Introduction of chemically accessible lewis basic sites inside mof channels. Cryst. Eng. Comm. 2016, 18, 3524–3550. [Google Scholar] [CrossRef]
- He, T.; Kong, X.-J.; Li, J.-R. Chemically stable metal-organic frameworks: Rational construction and application expansion. Acc. Chem. Res. 2021, 54, 3083–3094. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Shi, L.; Farha, O.K. Rational design of stable functional metal-organic frameworks. Mater. Horiz. 2023, 10, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tao, Y.; Wu, J.; Zhang, C.; He, H.; Long, L.; Lee, Y.; Li, T.; Zhang, Y.-B. Robust metal–triazolate frameworks for CO2 capture from flue gas. J. Am. Chem. Soc. 2020, 142, 2750–2754. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Lin, J.-B.; Shimizu, G.K.H.; Rajendran, A. Separation of CO2 and N2 on a hydrophobic metal organic framework CALF-20. Chem. Eng. J. 2022, 442, 136263. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Iremonger, S.S.; Dawson, K.W.; Shimizu, G.K. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 2009, 35, 5230–5232. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Nandi, S.; Nasa, P.; Vaidhyanathan, R. Enhancing the carbon capture capacities of a rigid ultra-microporous mof through gate-opening at low CO2 pressures assisted by swiveling oxalate pillars. Chem. Commun. 2016, 52, 1851–1854. [Google Scholar] [CrossRef]
- Peng, J.; Liu, Z.; Wu, Y.; Xian, S.; Li, Z. High-performance selective CO2 capture on a stable and flexible metal-organic framework via discriminatory gate-opening effect. ACS Appl. Mater. Interfaces 2022, 14, 21089–21097. [Google Scholar] [CrossRef]
- Ye, Y.; Xian, S.; Cui, H.; Tan, K.; Gong, L.; Liang, B.; Pham, T.; Pandey, H.; Krishna, R.; Lan, P.C.; et al. Metal-organic framework based hydrogen-bonding nanotrap for efficient acetylene storage and separation. J. Am. Chem. Soc. 2022, 144, 1681–1689. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, X.; Pham, T.; Verma, G.; Lan, P.C.; Shan, C.; Xing, H.; Forrest, K.A.; Suepaul, S.; Space, B.; et al. A MOF-based ultra-strong acetylene nano-trap for highly efficient C2H2/CO2 separation. Angew. Chem. Inter. Ed. 2021, 60, 5283–5288. [Google Scholar] [CrossRef]
- Wen, H.-M.; Liu, M.; Ling, Y.; Gu, X.-W.; Liu, D.; Yu, C.; Liang, Y.; Xie, B.; Li, B.; Hu, J. A metal-organic framework based propylene nano-trap with dual functionalities for highly efficient propylene/propane separation. J. Mater. Chem. A 2023, 11, 17821–17827. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Wang, X.; Fan, Z.-W.; Wang, H.-F.; Niu, Z.; Lang, J.-P. Design of a MOF-based nano-trap for the efficient separation of propane from propylene. Chem. Commum. 2023, 59, 5757–5760. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Z.; Lv, D.; Duan, C.; Sun, X.; Wu, H.; Shi, R.; Xia, Q.; Li, Z. Efficient adsorptive separation of C3H6 over C3H8 on flexible and thermoresponsive CPL-1. Chem. Eng. J. 2017, 328, 360–367. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, L.; Liu, X.; Wang, J.; Suo, X.; Chen, L.; Cui, X.; Xing, H. Ultramicroporous material based parallel and extended paraffin nano-trap for benchmark olefin purification. Nat. Commun. 2022, 13, 4928. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, S.A.; Su, J.; Zou, X.; Balkus, K.J., Jr. Carbonate-based zeolitic imidazolate framework for highly selective CO2 capture. Inorg. Chem. 2015, 54, 1816–1821. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Zhang, Y.-B.; Zhang, J.-P.; Chen, X.-M. Pillaring Zn-triazolate layers with flexible aliphatic dicarboxylates into three-dimensional metal-organic frameworks. Cryst. Growth Des. 2008, 8, 3673–3679. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.N.; Wain, D.L.; Hales, M.C. Infrared and infrared emission spectroscopy of the zinc carbonate mineral smithsonite. Spectrochim. Acta Part A 2008, 70, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Walton, K.S.; Sholl, D.S. Predicting multicomponent adsorption: 50 years of the ideal adsorbed solution theory. AIChE J. 2015, 61, 2757–2762. [Google Scholar] [CrossRef]
- Peng, J.; Zhong, J.; Liu, Z.; Xi, H.; Yan, J.; Xu, F.; Chen, X.; Wang, X.; Lv, D.; Li, Z. Multivariate metal-organic frameworks prepared by simultaneous metal/ligand exchange for enhanced c2–c3 selective recovery from natural gas. ACS Appl. Mater. Interfaces 2023, 15, 41466–41475. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhu, A.-X.; Lin, R.-B.; Qi, X.-L.; Chen, X.-M. Pore surface tailored sod-type metal-organic zeolites. Adv. Mater. 2011, 23, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ding, Q.; Hu, J.; Wang, Q.; Cui, X.; Xing, H. Selective capture of carbon dioxide from humid gases over a wide temperature range using a robust metal-organic framework. Chem. Eng. J. 2021, 405, 126937. [Google Scholar] [CrossRef]
- Xing, G.E.; Liu, Q.; Zhang, Y.; Zhang, S.; Dong, Y. Microporous zinc(ii) metal-organic framework with 6-connected pcu topology: Synthesis, structure, and gas adsorption properties. Z. Anorg. Allg. Chem. 2015, 641, 1556–1559. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Bai, N.; Li, S.N.; Bu, X.; Feng, P. Design of pore size and functionality in pillar-layered zn-triazolate-dicarboxylate frameworks and their high CO2/CH4 and C2 hydrocarbons/CH4 selectivity. Inorg. Chem. 2015, 54, 9862–9868. [Google Scholar] [CrossRef]
- Materials Studio, v7.0, Biovia Software Inc.: San Diego, CA, USA, 2013.
- Czepirski, L.; JagieŁŁo, J. Virial-type thermal equation of gas-solid adsorption. Chem. Eng. Sci. 1989, 44, 797–801. [Google Scholar] [CrossRef]
- Nuhnen, A.; Janiak, C. A practical guide to calculate the isosteric heat/enthalpy of adsorption via adsorption isotherms in metal-organic frameworks, mofs. Dalt. Trans. 2020, 49, 10295–10307. [Google Scholar] [CrossRef]









| MOFs | QCO2 at 15 kPa (cm3/g, STP) | QCO2 at 50 kPa (cm3/g, STP) | Qst (kJ/mol) | T (K) | Ref. |
|---|---|---|---|---|---|
| MAF-7 | 4.5 | 12.5 | 25 | 298 | [44] |
| ZnF(TZ) | 6.0 | 19.1 | 24 | 298 | [25] |
| ZnF(daTZ) | 21.4 | 34.1 | 33 | 298 | [25] |
| ZnDatzBdc | 1.9 | 5.8 | 29 | 298 | [30] |
| CALF-20 | 53.7 | 68.3 | 33.5 | 303 | [26] |
| ZnAtzOx | 60.5 | 65.0 | 55 | 303 | [29] |
| ZU-301 | 47.7 | 52.6 | 39 | 298 | [45] |
| Zn(FA)(datrz)2 | 12.3 | 28.5 | 24.0 | 298 | [46] |
| Zn2(TRZ)2(BDC) | 23.5 | 41.4 | - | 298 | [47] |
| Zn2(TRZ)2(FA) | 34.7 | 73.9 | - | 298 | [47] |
| ZnAtzCO3 | 44.8 | 56.0 | 32.6 | 298 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Fu, C.; Zhong, J.; Ye, B.; Xiao, J.; Duan, C.; Lv, D. Efficient Selective Capture of Carbon Dioxide from Nitrogen and Methane Using a Metal-Organic Framework-Based Nanotrap. Molecules 2023, 28, 7908. https://doi.org/10.3390/molecules28237908
Peng J, Fu C, Zhong J, Ye B, Xiao J, Duan C, Lv D. Efficient Selective Capture of Carbon Dioxide from Nitrogen and Methane Using a Metal-Organic Framework-Based Nanotrap. Molecules. 2023; 28(23):7908. https://doi.org/10.3390/molecules28237908
Chicago/Turabian StylePeng, Junjie, Chengmin Fu, Jiqin Zhong, Bin Ye, Jing Xiao, Chongxiong Duan, and Daofei Lv. 2023. "Efficient Selective Capture of Carbon Dioxide from Nitrogen and Methane Using a Metal-Organic Framework-Based Nanotrap" Molecules 28, no. 23: 7908. https://doi.org/10.3390/molecules28237908