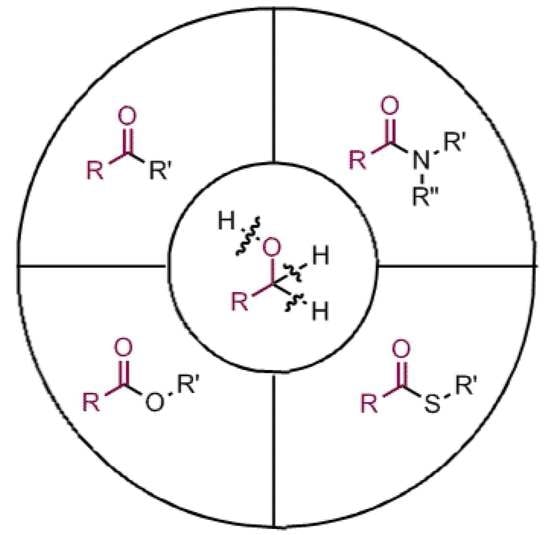
Progress in C-C and C-Heteroatom Bonds Construction Using Alcohols as Acyl Precursors
Abstract
:1. Introduction
2. C-C Bond Construction
3. C-O/C-S Bond Construction
4. C-N Bond Construction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, W.-W.; Liu, Q.; Ren, S.-Z.; Li, W.-Y.; Yi, L.-L.; Jing, H.; Sheng, L.-X.; Wan, Q.; Zhong, P.-F.; Fang, H.-L.; et al. The synthesis and evaluation of phenoxyacylhydroxamic acids as potential agents for Helicobacter pylori infections. Bioorg. Med. Chem. 2018, 26, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009. [Google Scholar] [CrossRef]
- Walter, M.W. Structure-based design of agrochemicals. Nat. Prod. Rep. 2002, 19, 278–291. [Google Scholar] [CrossRef]
- Dieter, R.K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Effenberger, F.; Epple, G. Catalytic Friedel-Crafts Acylation of Aromatic Compounds. Angew. Chem. Int. Ed. 1972, 11, 300–301. [Google Scholar] [CrossRef] [Green Version]
- Boon, J.A.; Levisky, J.A.; Pflug, J.L.; Wilkes, J.S. Friedel-Crafts reactions in ambient-temperature molten salts. J. Org. Chem. 1986, 51, 480–483. [Google Scholar] [CrossRef]
- Patil, M.L.; Borate, H.B.; Ponde, D.E.; Deshpande, V.H. Total synthesis of (±)-brasiliquinone B. Tetrahedron 2002, 58, 6615–6620. [Google Scholar] [CrossRef]
- Penteado, F.; Lopes, E.F.; Alves, D.; Perin, G.; Jacob, R.G.; Lenardão, E.J. α-Keto Acids: Acylating Agents in Organic Synthesis. Chem. Rev. 2019, 119, 7113–7278. [Google Scholar] [CrossRef]
- Tatamidani, H.; Yokota, K.; Kakiuchi, F.; Chatani, N. Catalytic Cross-Coupling Reaction of Esters with Organoboron Compounds and Decarbonylative Reduction of Esters with HCOONH4: A New Route to Acyl Transition Metal Complexes through the Cleavage of Acyl-Oxygen Bonds in Esters. J. Org. Chem. 2004, 69, 5615–5621. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Ghosh, K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Angew. Chem. Int. Ed. 2001, 40, 3458–3460. [Google Scholar] [CrossRef]
- Watson, A.J.A.; Williams, J.M.J. The Give and Take of Alcohol Activation. Science 2010, 329, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic Diamides and tert-Butoxide: Two Types of Ligands Enabling Practical Access to Alkyl Aryl Ethers via Cu-Catalyzed Coupling Reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, P.M.; Tassone, J.P.; Diaz, C.; Stradiotto, M. Exploiting Ancillary Ligation to Enable Nickel-Catalyzed C–O Cross-Couplings of Aryl Electrophiles with Aliphatic Alcohols. J. Am. Chem. Soc. 2018, 140, 5023–5027. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, C.; Gandhi, T. Alternative and Uncommon Acylating Agents—An Alive and Kicking Methodology. Chem. Asian J. 2021, 16, 2773–2794. [Google Scholar] [CrossRef]
- Huang, M.; Liu, J.; Li, Y.; Lan, X.-B.; Su, P.; Zhao, C.; Ke, Z. Recent advances on N-heterocyclic carbene transition metal complexes for dehydrogenative catalysis using alcohols. Catal. Today 2021, 370, 114–141. [Google Scholar] [CrossRef]
- Hartwig, J.F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 2011, 40, 1992–2002. [Google Scholar] [CrossRef]
- Kojima, T.; Mochizuki, M.; Takai, T.; Hoashi, Y.; Morimoto, S.; Seto, M.; Nakamura, M.; Kobayashi, K.; Sako, Y.; Tanaka, M.; et al. Discovery of 1,2,3,4-tetrahydropyrimido[1,2-a]benzimidazoles as novel class of corticotropin releasing factor 1 receptor antagonists. Bioorg. Med. Chem. 2018, 26, 2229–2250. [Google Scholar] [CrossRef] [PubMed]
- Torikai, K.; Koga, R.; Liu, X.; Umehara, K.; Kitano, T.; Watanabe, K.; Oishi, T.; Noguchi, H.; Shimohigashi, Y. Design and synthesis of benzoacridines as estrogenic and anti-estrogenic agents. Bioorg. Med. Chem. 2017, 25, 5216–5237. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Baran, P.S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 2011, 40, 1976–1991. [Google Scholar] [CrossRef]
- McMurray, L.; O’Hara, F.; Gaunt, M.J. Recent developments in natural product synthesis using metal-catalysed C–H bond functionalization. Chem. Soc. Rev. 2011, 40, 1885–1898. [Google Scholar] [CrossRef]
- Blangetti, M.; Rosso, H.; Prandi, C.; Deagostino, A.; Venturello, P. Suzuki-Miyaura cross coupling in acylation reactions, scope and recent developments. Molecules 2013, 18, 1188–1213. [Google Scholar] [CrossRef]
- Verheyen, T.; Turnhout, L.; Vandavasi, J.K.; Isbrandt, E.S.; De Borggraeve, W.M.; Newman, S.G. Ketone Synthesis by a Nickel-Catalyzed Dehydrogenative Cross-Coupling of Primary Alcohols. J. Am. Chem. Soc. 2019, 141, 6869–6874. [Google Scholar] [CrossRef]
- Suchand, B.; Sreenivasulu, C.; Satyanarayana, G. Palladium-Catalyzed Direct Oxidative Coupling of Iodoarenes with Primary Alcohols Leading to Ketones: Application to the Synthesis of Benzofuranones and Indenones. Eur. J. Org. Chem. 2019, 2019, 4832–4843. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Yan, Z.; Qiang, Q.; Huang, W.; Rong, Z.-Q. Redox-Neutral Nickel-Catalyzed Cross-Coupling Reactions of (Homo)allylic Alcohols and Aryltriflates. ACS Catal. 2021, 11, 7319–7326. [Google Scholar] [CrossRef]
- Xiao, F.; Shuai, Q.; Zhao, F.; Basle, O.; Deng, G.; Li, C.J. Palladium-Catalyzed Oxidative sp2 C-H Bond Acylation with Alcohols. Org. Lett. 2011, 13, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Li, F.; Shi, S.-L. Expedient Synthesis of Ketones via NHC/Nickel-Catalyzed Redox-Economical Coupling of Alcohols and Alkynes. Chin. J. Chem. 2020, 38, 1035–1039. [Google Scholar] [CrossRef]
- Yang, P.-F.; Shu, W. Direct Synthesis of Mono-α-arylated Ketones from Alcohols and Olefins via Ni-Catalyzed Oxidative Cross-Coupling. Org. Lett. 2020, 22, 6203–6208. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Li, G.; Xia, J.-B. Redox-Triggered Ruthenium-Catalyzed Remote C–H Acylation with Primary Alcohols. ACS Catal. 2020, 10, 12987–12995. [Google Scholar] [CrossRef]
- Spinello, B.J.; Wu, J.; Cho, Y.; Krische, M.J. Conversion of Primary Alcohols and Butadiene to Branched Ketones via Merged Transfer Hydrogenative Carbonyl Addition-Redox Isomerization Catalyzed by Rhodium. J. Am. Chem. Soc. 2021, 143, 13507–13512. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Kim, H.-K. Facile one-pot synthesis of ketones from primary alcohols under mild conditions. New J. Chem. 2021, 45, 13323–13328. [Google Scholar] [CrossRef]
- Otera, J.; Nishikido, J. Esterification: Methods, Reactions, and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Ogliaruso, M.A.; Wolfe, J.F. Synthesis of Carboxylic Acids, Esters and Their Derivatives; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Gowrisankar, S.; Neumann, H.; Beller, M. General and Selective Palladium-Catalyzed Oxidative Esterification of Alcohols. Angew. Chem. Int. Ed. 2011, 50, 5139–5143. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Meng, L.; Deng, Y.; Li, Y.; Lei, A. Palladium-Catalyzed Aerobic Oxidative Direct Esterification of Alcohols. Angew. Chem. Int. Ed. 2011, 50, 5144–5148. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F. A General and Efficient Zinc-Catalyzed Oxidation of Benzyl Alcohols to Aldehydes and Esters. Chem. Eur. J. 2012, 18, 8912–8915. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wang, X.; Lim, Y.N.; Kang, Y.-W.; Jang, H.-Y. N-Heterocyclic Carbene Catalysed Oxidative Coupling of Aldehydes with Alcohols/Thiols and One-Pot Oxidation/Esterification of Allylic Alcohols. Eur. J. Org. Chem. 2013, 2013, 7881–7885. [Google Scholar] [CrossRef]
- Song, T.; Park, J.E.; Chung, Y.K. Rhodium-Catalyzed Synthesis of Imines and Esters from Benzyl Alcohols and Nitroarenes: Change in Catalyst Reactivity Depending on the Presence or Absence of the Phosphine Ligand. J. Org. Chem. 2018, 83, 4197–4203. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, Y. Solvent-Controlled Copper-Catalyzed Oxidation of Benzylic Alcohols to Aldehydes and Esters. Eur. J. Org. Chem. 2013, 2013, 4503–4508. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, H.; Chai, W.; Zeng, X.; Xu, M.; Zhu, C.; Fu, R.; Yuan, R. Copper-Catalyzed Formation of C-O Bonds by Oxidative Coupling of Benzylic Alcohols with Ethers. Eur. J. Org. Chem. 2014, 2014, 6850–6853. [Google Scholar] [CrossRef]
- Ray, R.; Jana, R.D.; Bhadra, M.; Maiti, D.; Lahiri, G.K. Efficient and Simple Approaches Towards Direct Oxidative Esterification of Alcohols. Chem. Eur. J. 2014, 20, 15618–15624. [Google Scholar] [CrossRef]
- Hazra, S.; Deb, M.; Elias, A.J. Iodine catalyzed oxidation of alcohols and aldehydes to carboxylic acids in water: A metal-free route to the synthesis of furandicarboxylic acid and terephthalic acid. Green Chem. 2017, 19, 5548–5552. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, F.; Tao, C.; Yu, H.; Ruhlmann, L.; Wei, Y. Oxidative esterification of alcohols by a single-side organically decorated Anderson-type chrome-based catalyst. Green Chem. 2021, 23, 2652–2657. [Google Scholar] [CrossRef]
- Bonn, P.; Dreßler, D.; Weitenhagen, F.; Bolm, C. Mechanochemical Palladium-Catalyzed Oxidative Esterification of Alcohols. ACS Sustain. Chem. Eng. 2022, 10, 1361–1366. [Google Scholar] [CrossRef]
- Morino, Y.; Yatabe, T.; Suzuki, K.; Yamaguchi, K. Cu/N-Oxyl-catalyzed aerobic oxidative esterification to oxalic acid diesters from ethylene glycol via highly selective intermolecular alcohol oxidation. Green Chem. 2022, 24, 2017–2026. [Google Scholar] [CrossRef]
- Srimani, D.; Balaraman, E.; Gnanaprakasam, B.; Ben-David, Y.; Milstein, D. Ruthenium Pincer-Catalyzed Cross-Dehydrogenative Coupling of Primary Alcohols with Secondary Alcohols under Neutral Conditions. Adv. Synth. Catal. 2012, 354, 2403–2406. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Wang, C.; Li, J.; Jiang, X.; Wei, Y.; Tang, W.; Xue, D.; Xiao, J. Chemoselective dehydrogenative esterification of aldehydes and alcohols with a dimeric rhodium(II) catalyst. Chem. Sci. 2016, 7, 4428–4434. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.H.; Trivelli, X.; Capet, F.; Paul, J.-F.; Dumeignil, F.; Gauvin, R.M. Manganese Pincer Complexes for the Base-Free, Acceptorless Dehydrogenative Coupling of Alcohols to Esters: Development, Scope, and Understanding. ACS Catal. 2017, 7, 2022–2032. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Zhao, D.; Li, Y. Aldehyde Effect and Ligand Discovery in Ru-Catalyzed Dehydrogenative Cross-Coupling of Alcohols to Esters. Chem. Commun. 2019, 55, 2797–2800. [Google Scholar] [CrossRef]
- Zhao, L.; He, X.; Cui, T.; Nie, X.; Xu, J.; Zheng, X.; Jiang, W.; Yuan, M.; Chen, H.; Fu, H.; et al. Acceptorless Dehydrogenative Cross-Coupling of Primary Alcohols Catalyzed by an N-Heterocyclic Carbene-Nitrogen-Phosphine Chelated Ruthenium(II) Complex. J. Org. Chem. 2022, 87, 4550–4559. [Google Scholar] [CrossRef]
- Sarbajna, A.; Dutta, I.; Daw, P.; Dinda, S.; Rahaman, S.M.W.; Sarkar, A.; Bera, J.K. Catalytic Conversion of Alcohols to Carboxylic Acid Salts and Hydrogen with Alkaline Water. ACS Catal. 2017, 7, 2786–2790. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.-Q.; Cheng, H.; Zheng, Z.-H.; Yuan, Y.; Chen, C.; Verpoort, F. Gram-scale synthesis of carboxylic acids via catalytic acceptorless dehydrogenative coupling of alcohols and hydroxides at an ultralow Ru loading. Appl. Catal. A 2022, 630, 118443. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, Y.; Mao, H.; Cheng, Y.; Zhu, C. Tetraethylammonium Bromide-Catalyzed Oxidative Thioesterification of Aldehydes and Alcohols. Adv. Synth. Catal. 2013, 355, 3558–3562. [Google Scholar] [CrossRef]
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley-Interscience: New York, NY, USA, 2000. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.M.; Suppo, J.S.; Campagne, J.M. Nonclassical Routes for Amide Bond Formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 2014, 43, 2714–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Ojeda-Porras, A.; Gamba-Sánchez, D. Recent Developments in Amide Synthesis Using Nonactivated Starting Materials. J. Org. Chem. 2016, 81, 11548–11555. [Google Scholar] [CrossRef]
- Wu, X.-F.; Sharif, M.; Pews-Davtyan, A.; Langer, P.; Ayub, K.; Beller, M. The First Zn II -Catalyzed Oxidative Amidation of Benzyl Alcohols with Amines under Solvent-Free Conditions. Eur. J. Org. Chem. 2013, 2013, 2783–2787. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Ngiam, J.S.Y.; Seayad, A.M.; Tuan, D.T.; Johannes, C.W.; Chen, A. Tandem oxidative amidation of benzyl alcohols with amine hydrochloride salts catalysed by iron nitrate. Tetrahedron Lett. 2013, 54, 4922–4925. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hull, K.L. Rhodium-Catalyzed Oxidative Amidation of Sterically Hindered Aldehydes and Alcohols. ACS Catal. 2016, 6, 8214–8218. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Ganjbakhsh, N.; Tavana, M.M.; Boukherroub, R. Ultrasound-assisted direct oxidative amidation of benzyl alcohols catalyzed by graphite oxide. Ultrason. Sonochem. 2016, 32, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Yang, Y.; Feng, W.; Ge, Q.; Feng, Y.; Zeng, X.; Chai, W.; Yi, J.; Yuan, R. An efficient, eco-friendly and sustainable tandem oxidative amidation of alcohols with amines catalyzed by heteropolyanion-based ionic liquids via a bifunctional catalysis process. Tetrahedron 2016, 72, 8319–8326. [Google Scholar] [CrossRef]
- Zultanski, S.L.; Zhao, J.; Stahl, S.S. Practical Synthesis of Amides via Copper/ABNO-Catalyzed Aerobic Oxidative Coupling of Alcohols and Amines. J. Am. Chem. Soc. 2016, 138, 6416–6419. [Google Scholar] [CrossRef] [Green Version]
- Piszel, P.E.; Vasilopoulos, A.; Stahl, S.S. Oxidative Amide Coupling from Functionally Diverse Alcohols and Amines using Aerobic Copper/Nitroxyl Catalysis. Angew. Chem. Int. Ed. 2019, 58, 12211–12215. [Google Scholar] [CrossRef]
- Kataoka, K.; Wachi, K.; Jin, X.; Suzuki, K.; Sasano, Y.; Iwabuchi, Y.; Hasegawa, J.; Mizuno, N.; Yamaguchi, K. CuCl/TMEDA/nor-AZADO-catalyzed aerobic oxidative acylation of amides with alcohols to produce imides. Chem. Sci. 2018, 9, 4756–4768. [Google Scholar] [CrossRef] [Green Version]
- Krabbe, S.W.; Chan, V.S.; Franczyk, T.S.; Shekhar, S.; Napolitano, J.G.; Presto, C.A.; Simanis, J.A. Copper-Catalyzed Aerobic Oxidative Amidation of Benzyl Alcohols. J. Org. Chem. 2016, 81, 10688–10697. [Google Scholar] [CrossRef]
- Balaboina, R.; Thirukovela, N.S.; Vadde, R.; Vasam, C.S. Amide bond synthesis via silver(I) N-heterocyclic carbene-catalyzed and tert-butyl hydroperoxide-mediated oxidative coupling of alcohols with amines under base free conditions. Tetrahedron Lett. 2019, 60, 847–851. [Google Scholar] [CrossRef]
- Stephenson, C.R.J.; Yoon, T.P.; MacMillan, D.W.C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Liu, Q.; Wu, L.-Z. Recent advances in visible-light-driven organic reactions. Natl. Sci. Rev. 2017, 4, 359–380. [Google Scholar] [CrossRef] [Green Version]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Shee, M.; Venkatesh, Y.; Singh, A.K.; Samanta, S.; Singh, N.D.P. Organophotoredox Mediated Amide Synthesis by Coupling Alcohol and Amine via Aerobic Oxidation of Alcohol. Chem. Eur. J. 2020, 26, 3703–3708. [Google Scholar] [CrossRef]
- Gaspa, S.; Farina, A.; Tilocca, M.; Porcheddu, A.; Pisano, L.; Carraro, M.; Azzena, U.; De Luca, L. Visible-Light Photoredox-Catalyzed Amidation of Benzylic Alcohols. J. Org. Chem. 2020, 85, 11679–11687. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Ghosh, S.C.; Panda, A.B. Visible Light-Driven Efficient Synthesis of Amides from Alcohols using Cu–N–TiO2 Heterogeneous Photocatalyst. Eur. J. Org. Chem. 2021, 2021, 657–662. [Google Scholar] [CrossRef]
- Chen, C.; Hong, S.H. Oxidative amide synthesis directly from alcohols with amines. Org. Biomol. Chem. 2011, 9, 20–26. [Google Scholar] [CrossRef]
- Chen, C.; Verpoort, F.; Wu, Q. Atom-economic dehydrogenative amide synthesis via ruthenium catalysis. RSC Adv. 2016, 6, 55599–55607. [Google Scholar] [CrossRef]
- Heravi, M.R.P.; Hosseinian, A.; Rahmani, Z.; Ebadi, A.; Vessally, E. Transition-metal-catalyzed dehydrogenative coupling of alcohols and amines: A novel and atom-economical access to amides. J. Chin. Chem. Soc. 2021, 68, 723–737. [Google Scholar] [CrossRef]
- Gunanathan, C.; Ben-David, Y.; Milstein, D. Direct Synthesis of Amides from Alcohols and Amines with Liberation of H2. Science 2007, 317, 790–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Ghosh, S.C.; Li, Y.; Hong, S.H. Well-Defined N-Heterocyclic Carbene Based Ruthenium Catalysts for Direct Amide Synthesis from Alcohols and Amines. Organometallics 2010, 29, 1374–1378. [Google Scholar] [CrossRef]
- Dam, J.H.; Osztrovszky, G.; Nordstrøm, L.U.; Madsen, R. Amide Synthesis from Alcohols and Amines Catalyzed by Ruthenium N-Heterocyclic Carbene Complexes. Chem. Eur. J. 2010, 16, 6820–6827. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Hong, S.H. N-Heterocyclic Carbene Based Ruthenium-Catalyzed Direct Amide Synthesis from Alcohols and Secondary Amines: Involvement of Esters. J. Org. Chem. 2011, 76, 10005–10010. [Google Scholar] [CrossRef]
- Kim, K.; Kang, B.; Hong, S.H. N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron 2015, 71, 4565–4569. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, M.-Q.; Cheng, C.-X.; Wang, H.-J.; Lu, Q.; Liu, H.-F.; Yao, F.-B.; Verpoort, F.; Chen, C. In situ generated ruthenium catalytic systems bearing diverse N-heterocyclic carbene precursors for the atom-economic amide synthesis from alcohols and amines. Chem. Asian J. 2018, 13, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.-Q.; Zhang, N.; Wang, H.-J.; Miao, Y.; Su, W.; Yuan, Y.; Chen, C.; Verpoort, F. Efficient N-heterocyclic carbene/ruthenium catalytic systems for the alcohol amidation with amines: Involvement of poly-carbene complexes? Chemcatchem 2018, 10, 4338–4345. [Google Scholar] [CrossRef]
- Wu, X.-J.; Wang, H.-J.; Yang, Z.-Q.; Tang, X.-S.; Yuan, Y.; Su, W.; Chen, C.; Verpoort, F. Efficient and phosphine-free bidentate N-heterocyclic carbene/ruthenium catalytic systems for the dehydrogenative amidation of alcohols and amines. Org. Chem. Front. 2019, 6, 563–570. [Google Scholar] [CrossRef]
- Chen, C.; Miao, Y.; De Winter, K.; Wang, H.-J.; Demeyere, P.; Yuan, Y.; Verpoort, F. Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules 2018, 23, 2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.-Q.; Wang, Z.-Q.; Sang, W.; Zhang, R.; Cheng, H.; Chen, C.; Peng, D.-Y. Dehydrogenative amide synthesis from alcohols and amines utilizing N-heterocyclic carbene-based ruthenium complexes as efficient catalysts: The influence of catalyst loadings, ancillary and added ligands. Polyhedron 2021, 195, 114979. [Google Scholar] [CrossRef]
- Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H. Oxidative Synthesis of Amides and Pyrroles via Dehydrogenative Alcohol Oxidation by Ruthenium Diphosphine Diamine Complexes. Organometallics 2011, 30, 4174–4179. [Google Scholar] [CrossRef]
- Srimani, D.; Balaraman, E.; Hu, P.; Ben-David, Y.; Milstein, D. Formation of Tertiary Amides and Dihydrogen by Dehydrogenative Coupling of Primary Alcohols with Secondary Amines Catalyzed by Ruthenium Bipyridine-Based Pincer Complexes. Adv. Synth. Catal. 2013, 355, 2525–2530. [Google Scholar] [CrossRef]
- Saha, B.; Sengupta, G.; Sarbajna, A.; Dutta, I.; Bera, J.K. Amide synthesis from alcohols and amines catalyzed by a Ru(II)-N-heterocyclic carbene (NHC)-carbonyl complex. J. Organomet. Chem. 2014, 771, 124–130. [Google Scholar] [CrossRef]
- Watson, A.J.A.; Wakeham, R.J.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalysed oxidation of alcohols to amides using a hydrogen acceptor. Tetrahedron 2014, 70, 3683–3690. [Google Scholar] [CrossRef]
- Sindhuja, E.; Ramesh, R.; Balaji, S.; Liu, Y. Direct Synthesis of Amides from Coupling of Alcohols and Amines Catalyzed by Ruthenium(II) Thiocarboxamide Complexes under Aerobic Conditions. Organometallics 2014, 33, 4269–4278. [Google Scholar] [CrossRef]
- Higuchi, T.; Tagawa, R.; Iimuro, A.; Akiyama, S.; Nagae, H.; Mashima, K. Tunable Ligand Effects on Ruthenium Catalyst Activity for Selectively Preparing Imines or Amides by Dehydrogenative Coupling Reactions of Alcohols and Amines. Chem. Eur. J. 2017, 23, 12795–12804. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Xie, Y.; Zhou, Q.Q.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Near-Ambient-Temperature Dehydrogenative Synthesis of the Amide Bond: Mechanistic Insight and Applications. ACS Catal. 2021, 11, 7383–7393. [Google Scholar] [CrossRef]
- Egly, J.; Chen, W.; Maisse-François, A.; Bellemin-Laponnaz, S.; Achard, T. Half-Sandwich Ruthenium Complexes Bearing Hemilabile κ2-(C,S)—Thioether-Functionalized NHC Ligands: Application to Amide Synthesis from Alcohol and Amine. Eur. J. Inorg. Chem. 2022, 2022, e20210103. [Google Scholar] [CrossRef]
- Zheng, Y.; Nie, X.; Long, Y.; Ji, L.; Fu, H.; Zheng, X.; Chen, H.; Li, R. Ruthenium-Catalyzed Synthesis of N-substituted Lactams by Acceptorless Dehydrogenative Coupling of Diols with Primary Amines. Chem. Commun. 2019, 55, 12384–12387. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Q.-Q.; Montag, M.; Ben-David, Y.; Milstein, D. Acceptorless dehydrogenative synthesis of primary amides from alcohols and ammonia. Chem. Sci. 2022, 13, 3894–3901. [Google Scholar] [CrossRef]
- Vasseur, A.; Bruffaerts, J.; Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 2016, 8, 209–219. [Google Scholar] [CrossRef]
- Sommer, H.; Juliá-Hernández, F.; Martin, R.; Marek, I. Walking Metals for Remote Functionalization. ACS Cent. Sci. 2018, 4, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, N.; Wang, X.; Wang, Z.; Liu, F.; Rong, Z.-Q. Redox-neutral remote amidation of alkenyl alcohols via long-range isomerization/transformation. Org. Chem. Front. 2022, 9, 5942–5948. [Google Scholar] [CrossRef]
- Peña-López, M.; Neumann, H.; Beller, M. Iron(II) Pincer-Catalyzed Synthesis of Lactones and Lactams through a Versatile Dehydrogenative Domino Sequence. Chemcatchem 2015, 7, 865–871. [Google Scholar] [CrossRef]
- Lane, E.M.; Uttley, K.B.; Hazari, N.; Bernskoetter, W. Iron-Catalyzed Amide Formation from the Dehydrogenative Coupling of Alcohols and Secondary Amines. Organometallics 2017, 36, 2020–2025. [Google Scholar] [CrossRef]
- Kumar, A.; Espinosa-Jalapa, N.A.; Leitus, G.; Diskin-Posner, Y.; Avram, L.; Milstein, D. Direct Synthesis of Amides by Dehydrogenative Coupling of Amines with either Alcohols or Esters: Manganese Pincer Complex as Catalyst. Angew. Chem. Int. Ed. 2017, 56, 14992–14996. [Google Scholar] [CrossRef] [PubMed]
- Zubar, V.; Brzozowska, A.; Sklyaruk, J.; Rueping, M. Dehydrogenative and Redox-Neutral N-Heterocyclization of Aminoalcohols Catalyzed by Manganese Pincer Complexes. Organometallics 2022, 41, 1743–1747. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, F.; Wang, X.; Qiang, Q.; Li, Y.; Zhang, Y.; Rong, Z.-Q. Redox-neutral dehydrogenative cross-coupling of alcohols and amines enabled by nickel catalysis. Org. Chem. Front. 2022, 9, 1703–1710. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Y.; Tang, C.; Deng, G.-J. Peroxide-Mediated Transition-Metal-Free Direct Amidation of Alcohols with Nitroarenes. Org. Lett. 2012, 14, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Dong, Y.; Xu, B.; Li, Y.; Zeng, R. Mild Amide Synthesis Using Nitrobenzene under Neutral Conditions. Org. Lett. 2022, 24, 4766–4771. [Google Scholar] [CrossRef]
- Kang, B.; Fu, Z.; Hong, S.H. Ruthenium-Catalyzed Redox-Neutral and Single-Step Amide Synthesis from Alcohol and Nitrile with Complete Atom Economy. J. Am. Chem. Soc. 2013, 135, 11704–11707. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, M.; Xia, Z.; Tan, Z.; Deng, W.; Fang, C. Direct Synthesis of Amides from Benzonitriles and Benzylic Alcohols via a KOt-Bu-Mediated MPV-type Hydrogen Transfer Process. J. Org. Chem. 2022, 87, 8884–8891. [Google Scholar] [CrossRef]

















































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Tan, B.; Li, Q.; Tan, Q.; Huang, H. Progress in C-C and C-Heteroatom Bonds Construction Using Alcohols as Acyl Precursors. Molecules 2022, 27, 8977. https://doi.org/10.3390/molecules27248977
Zhao F, Tan B, Li Q, Tan Q, Huang H. Progress in C-C and C-Heteroatom Bonds Construction Using Alcohols as Acyl Precursors. Molecules. 2022; 27(24):8977. https://doi.org/10.3390/molecules27248977
Chicago/Turabian StyleZhao, Feng, Bin Tan, Qing Li, Qi Tan, and Huawen Huang. 2022. "Progress in C-C and C-Heteroatom Bonds Construction Using Alcohols as Acyl Precursors" Molecules 27, no. 24: 8977. https://doi.org/10.3390/molecules27248977