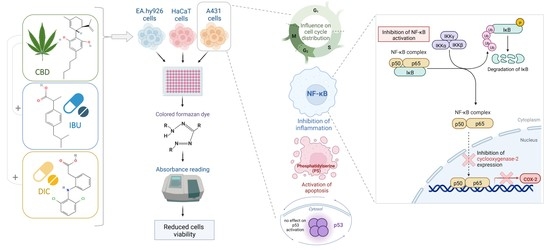
Cannabidiol and Its Combinations with Nonsteroidal Anti-Inflammatory Drugs Induce Apoptosis and Inhibit Activation of NF-κB Signaling in Vulvar Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. CBD and NSAIDs Influence the Viability of A431, HaCaT and EA.hy926 Cells
2.2. CBD and NSAIDs Induce Apoptotic Cell Death in VSCC Cells, but It Is Not Accompanied by the Increased p53 Level
2.3. The Distribution of Cell Cycle Phases Remains Unaltered after the Treatment with CBD and NSAIDs
2.4. The Translocation of NF-κB to the Nucleus Is Diminished after the Treatment with CBD and Its Combination with NSAIDs
2.5. The Activation of NF-κB Signaling Is Reduced after the Treatment with CBD and Its Combination with NSAIDs
2.6. COX-2 Expression Is Diminished after the Treatment with CBD and Its Combinations with DIC and IBU
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Viability Assay
4.2. Apoptosis Analysis
4.3. Cell Cycle Assay
4.4. Nuclear, Cytosolic and Total Protein Lysates Preparation
4.5. Western Blot Analysis
4.6. NF-ĸB Binding Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From Inflammation to Cancer. Immun. Ageing 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Muigai, J.; Jacob, L.; Dinas, K.; Kostev, K.; Kalder, M. Potential Delay in the Diagnosis of Vulvar Cancer and Associated Risk Factors in Women Treated in German Gynecological Practices. Oncotarget 2018, 9, 8725–8730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scampa, M.; Kalbermatten, D.F.; Oranges, C.M. Squamous Cell Carcinoma of the Vulva: A Survival and Epidemiologic Study with Focus on Surgery and Radiotherapy. J. Clin. Med. 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.; Wong, V.; Brooks, J.; Kroumpouzos, G. Genital Diseases in the Mature Woman. Clin. Dermatol. 2018, 36, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Hellman, K.; Holmberg, E.; Bjurberg, M.; Borgfeldt, C.; Dahm-Kähler, P.; Flöter Rådestad, A.; Hjerpe, E.; Högberg, T.; Marcickiewicz, J.; Rosenberg, P.; et al. Primary Treatment and Relative Survival by Stage and Age in Vulvar Squamous Cell Carcinoma: A Population-Based SweGCG Study. Gynecol. Oncol. 2020, 159, 663–671. [Google Scholar] [CrossRef]
- Rottmann, M.; Beck, T.; Burges, A.; Dannecker, C.; Kiechle, M.; Mayr, D.; Schlesinger-Raab, A.; Schubert-Fritschle, G.; Engel, J. Trends in Surgery and Outcomes of Squamous Cell Vulvar Cancer Patients over a 16-Year Period (1998–2013): A Population-Based Analysis. J. Cancer Res. Clin. Oncol. 2016, 142, 1331–1341. [Google Scholar] [CrossRef]
- Buchholz, A.; Vattai, A.; Fürst, S.; Vilsmaier, T.; Kuhn, C.; Schmoeckel, E.; Mayr, D.; Dannecker, C.; Mahner, S.; Jeschke, U.; et al. EP4 as a Negative Prognostic Factor in Patients with Vulvar Cancer. Cancers 2021, 13, 1410. [Google Scholar] [CrossRef]
- Milliken, S.; May, J.; Sanderson, P.A.; Congiu, M.A.; D’Oria, O.; Golia D’Augè, T.; Caruso, G.; DI Donato, V.; Benedetti Panici, P.; Giannini, A. Reducing the Radicality of Surgery for Vulvar Cancer: Are Smaller Margins Safer? Minerva Obstet. Gynecol. 2021, 73, 160–165. [Google Scholar] [CrossRef]
- Wang, S.; Sparks, A.D.; Rao, Y.J.; Long, B. Incidence, Mortality, and Treatment Patterns of Synchronous Lower Genital Tract Squamous Cell Carcinoma. J. Low. Genit. Tract Dis. 2022, 26, 202–206. [Google Scholar] [CrossRef]
- Papierska, K.; Krajka-Kuźniak, V.; Kleszcz, R.; Stefański, T.; Kurczab, R.; Kubicki, M. The Synthesis of Novel Thioderivative Chalcones and Their Influence on NF-ΚB, STAT3 and NRF2 Signaling Pathways in Colorectal Cancer Cells. Sci. Rep. 2022, 12, 14915. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Seyed Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.o.; Hushmandi, K.; et al. Regulation of Nuclear Factor-KappaB (NF-ΚB) Signaling Pathway by Non-Coding RNAs in Cancer: Inhibiting or Promoting Carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.K.; Sharma, N.; Lee, H.B.; Nongalleima, K.; Sharma, M. Novel Dual Inhibitors of Nuclear Factor-Kappa B (NF-κ B) and Cyclooxygenase- 2 (COX-2): Synthesis, In Vitro Anticancer Activity and Stability Studies of Lantadene–Non Steroidal Anti-Inflammatory Drug (NSAID) Conjugates. Curr. Top. Med. Chem. 2014, 14, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Raspollini, M.R.; Asirelli, G.; Taddei, G.L. The Role of Angiogenesis and COX-2 Expression in the Evolution of Vulvar Lichen Sclerosus to Squamous Cell Carcinoma of the Vulva. Gynecol. Oncol. 2007, 106, 567–571. [Google Scholar] [CrossRef]
- Luschnig, P.; Schicho, R. Cannabinoids in Gynecological Diseases. Med. Cannabis Cannabinoids 2019, 2, 14–21. [Google Scholar] [CrossRef]
- Razlog, R.; Kruger, C.A.; Abrahamse, H. Enhancement of Conventional and Photodynamic Therapy for Treatment of Cervical Cancer with Cannabidiol. Integr. Cancer Ther. 2022, 21, 15347354221092706. [Google Scholar] [CrossRef]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Inkol, J.M.; Hocker, S.E.; Mutsaers, A.J. Combination Therapy with Cannabidiol and Chemotherapeutics in Canine Urothelial Carcinoma Cells. PLoS ONE 2021, 16, e0255591. [Google Scholar] [CrossRef]
- Griffiths, C.; Aikins, J.; Ostrovsky, O.; Warshal, D. Cannabidiol Suppresses 3-Dimensional Ovarian Cancer Growth and May Enhance Potency of Classic and Epigenetic Therapies. Gynecol. Oncol. 2021, 162, S102–S103. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-Induced Cell Death in Endometrial Cancer Cells: Involvement of TRPV1 Receptors in Apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory Feedback Control of NF-ΚB Signalling in Health and Disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Cykowiak, M.; Baer-Dubowska, W. Phytochemical Combinations Modulate the Activation of Nrf2 and Expression of SOD in Pancreatic Cancer Cells More Efficiently Than Single Plant Components. Proceedings 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Cykowiak, M.; Kleszcz, R.; Kucińska, M.; Paluszczak, J.; Szaefer, H.; Plewiński, A.; Piotrowska-Kempisty, H.; Murias, M.; Krajka-Kuźniak, V. Attenuation of Pancreatic Cancer In Vitro and In Vivo via Modulation of Nrf2 and NF-ΚB Signaling Pathways by Natural Compounds. Cells 2021, 10, 3556. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, X.; Jeschke, U.; von Schönfeldt, V. COX-2-PGE2-EPs in Gynecological Cancers. Arch. Gynecol. Obstet. 2020, 301, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Misiorek, J.O.; Kruhlenia, N.; Przybyl, L.; Kleszcz, R.; Rolle, K.; Krajka-Kuźniak, V. COXIBs and 2,5-Dimethylcelecoxib Counteract the Hyperactivated Wnt/β-Catenin Pathway and COX-2/PGE2/EP4 Signaling in Glioblastoma Cells. BMC Cancer 2021, 21, 493. [Google Scholar] [CrossRef]
- Narożna, M.; Krajka-Kuźniak, V.; Kleszcz, R.; Baer-Dubowska, W. Indomethacin and Diclofenac Hybrids with Oleanolic Acid Oximes Modulate Key Signaling Pathways in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1230. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.R.; Sun, J.; Vachani, A.; Wallace, A.F.; Silverberg, M.; Kapoor, V.; Albelda, S.M. Cycloxygenase-2 Inhibition Augments the Efficacy of a Cancer Vaccine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, Y.; Pandit, A.; Swarnakar, S. Insights Into the Regulation of Gynecological Inflammation-Mediated Malignancy by Metalloproteinases. Front. Cell Dev. Biol. 2021, 9, 3408. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia Glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Kleszcz, R.; Studzińska-Sroka, E.; Łukaszyk, A.; Szoszkiewicz, A.; Stelcer, E.; Jopek, K.; Rucinski, M.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Lichen Secondary Metabolites Inhibit the Wnt/β-Catenin Pathway in Glioblastoma Cells and Improve the Anticancer Effects of Temozolomide. Cells 2022, 11, 1084. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Kleszcz, R.; Stasiłowicz-Krzemień, A.; Cielecka-Piontek, J. Sodium Butyrate Enhances Curcuminoids Permeability through the Blood-Brain Barrier, Restores Wnt/β-Catenin Pathway Antagonists Gene Expression and Reduces the Viability of Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11285. [Google Scholar] [CrossRef]
- Taylor, A.H.; Tortolani, D.; Ayakannu, T.; Konje, J.C.; Maccarrone, M. (Endo)Cannabinoids and Gynaecological Cancers. Cancers 2020, 13, 37. [Google Scholar] [CrossRef]
- Reiss, M.; Brash, D.E.; Muñoz-Antonia, T.; Simon, J.A.; Ziegler, A.; Vellucci, V.F.; Zhou, Z.L. Status of the P53 Tumor Suppressor Gene in Human Squamous Carcinoma Cell Lines. Oncol. Res. 1992, 4, 349–357. [Google Scholar]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-Induced Apoptosis Is Mediated by Activation of Noxa in Human Colorectal Cancer Cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of P22phox and Nox4 Expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids Induce Apoptosis of Pancreatic Tumor Cells via Endoplasmic Reticulum Stress–Related Genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid Action Induces Autophagy-Mediated Cell Death through Stimulation of ER Stress in Human Glioma Cells. J. Clin. Invest. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbini, L.F.; Wang, Y.; Cho, J.-Y.; Libermann, T.A. Constitutive Activation of Nuclear Factor ΚB P50/P65 and Fra-1 and JunD Is Essential for Deregulated Interleukin 6 Expression in Prostate Cancer1. Cancer Res. 2003, 63, 2206–2215. [Google Scholar]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.-W. NF-ΚB in Cancer: From Innocent Bystander to Major Culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-ΚB Regulation in the Immune System. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Narozna, M.; Krajka-Kuzniak, V.; Kleszcz, R.; Baer-Dubowska, W. Abstract 106: Diclofenac Hybrids with Oleanolic Acid Oximes Modulate Key Signaling Pathways in Pancreatic Cancer Cells. Cancer Res. 2022, 82, 106. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Fatalska, A.; Rusetska, N.; Bakuła-Zalewska, E.; Kowalik, A.; Zięba, S.; Wroblewska, A.; Zalewski, K.; Goryca, K.; Domański, D.; Kowalewska, M. Inflammatory Proteins HMGA2 and PRTN3 as Drivers of Vulvar Squamous Cell Carcinoma Progression. Cancers 2020, 13, 27. [Google Scholar] [CrossRef]
- Pęczek, P.; Gajda, M.; Rutkowski, K.; Fudalej, M.; Deptała, A.; Badowska-Kozakiewicz, A.M. Cancer-Associated Inflammation: Pathophysiology and Clinical Significance. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Clancy, A.A.; Spaans, J.N.; Weberpals, J.I. The Forgotten Woman’s Cancer: Vulvar Squamous Cell Carcinoma (VSCC) and a Targeted Approach to Therapy. Ann. Oncol. 2016, 27, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Ewing-Graham, P.C.; Van Den Bosch, T.P.P.; Swagemakers, S.M.A.; Santegoets, L.A.M.; Van Doorn, H.C.; Van Der Spek, P.J.; Koljenović, S.; Van Kemenade, F.J. Nuclear Factor IB Is Downregulated in Vulvar Squamous Cell Carcinoma (VSCC): Unravelling Differentially Expressed Genes in VSCC through Gene Expression Dataset Analysis. Oncol. Lett. 2021, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Voss, F.O.; van Beurden, M.; Jordanova, E.S. Topical Imiquimod as First-Line Treatment for Vulvar Intraepithelial Neoplasia. Lancet 2022, 399, 1755–1757. [Google Scholar] [CrossRef] [PubMed]







| Compound/Combination | A431 [IC50 ± SEM (µM)] | HaCaT [IC50 ± SEM (µM)] | EA.hy926 [IC50 ± SEM (µM)] |
|---|---|---|---|
| CBD | 30.0 ± 0.28 | 31.0 ± 1.94 | 35.5 ± 3.24 |
| DIC | >50 | >50 | >50 |
| CBD + DIC | 34.0 ± 1.4 | 37.5 ± 3.86 | 35.0 ± 1.65 |
| IBU | >50 | >50 | >50 |
| CBD + IBU | 17.5 ± 2.69 | 31.0 ± 2.98 | 25.0 ± 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajka-Kuźniak, V.; Papierska, K.; Narożna, M.; Jelińska, A.; Majchrzak-Celińska, A. Cannabidiol and Its Combinations with Nonsteroidal Anti-Inflammatory Drugs Induce Apoptosis and Inhibit Activation of NF-κB Signaling in Vulvar Squamous Cell Carcinoma. Molecules 2022, 27, 8779. https://doi.org/10.3390/molecules27248779
Krajka-Kuźniak V, Papierska K, Narożna M, Jelińska A, Majchrzak-Celińska A. Cannabidiol and Its Combinations with Nonsteroidal Anti-Inflammatory Drugs Induce Apoptosis and Inhibit Activation of NF-κB Signaling in Vulvar Squamous Cell Carcinoma. Molecules. 2022; 27(24):8779. https://doi.org/10.3390/molecules27248779
Chicago/Turabian StyleKrajka-Kuźniak, Violetta, Katarzyna Papierska, Maria Narożna, Anna Jelińska, and Aleksandra Majchrzak-Celińska. 2022. "Cannabidiol and Its Combinations with Nonsteroidal Anti-Inflammatory Drugs Induce Apoptosis and Inhibit Activation of NF-κB Signaling in Vulvar Squamous Cell Carcinoma" Molecules 27, no. 24: 8779. https://doi.org/10.3390/molecules27248779