Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept
Abstract
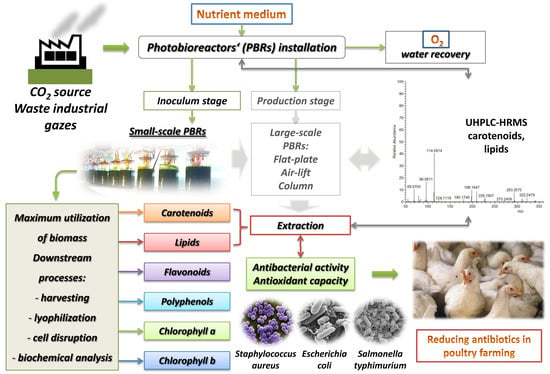
:1. Introduction
2. Results
2.1. Growth Rate and Phytochemical Analysis of S. obliquus Biomass Grown in Innovative Small-Scale PBRs under Internal (Red or Green) and External White LED Light
- For SC-PBR1:
- For SC-PBR2:
2.2. Antimicrobial Activity of S. obliquus Extracts
2.3. Combination Effects between Dichloromethane Extracts of S. obliquus Biomass and Penicillin, Fluoroquinolones or Oregano Essential Oil
2.4. Antioxidant Capacity
E2 = 0.1385 ± 0.0024 mmol TE/g
2.5. In Vitro Cytotoxicity of S. obliquus Dichloromethane Extracts
3. Discussion
4. Materials and Methods
4.1. Algae Strain, Medium and Cultural Conditions
4.2. Dry Weight Estimation, Calculation of Specific Growth Rate, Lyophilization of Microalgal Biomass and Preparation of Dichloromethane Extracts
4.3. Quantitative Determination of Polyphenols in S. obliquus Biomass
4.4. Quantitative Determination of Flavonoids in S. obliquus Biomass
4.5. Quantitative Determination of Chlorophylls and Carotenoids in S. obliquus Biomass
4.6. Quantitative Determination of Lipids in S. obliquus Biomass
4.7. UHPLC–HRMS
4.8. Distillation of Oregano Oil
4.9. Bacterial Strains and Culture Conditions
4.10. Determination of Minimal Inhibitory Concentrations
4.11. Checkerboard Assay
- Step 1
- Step 2
4.12. Metabolic (Cell Redox, Respiratory and Dehydrogenase) Activity Assay
4.13. Cell Viability Assay
4.14. Cupric Ion-Reducing Antioxidant Capacity (CUPRAC) Assay
- (1)
- 10 mM of CuCl2 in distilled H2O;
- (2)
- 1.0 M of ammonium acetate buffer; pH7;
- (3)
- 7.5 mM of neocuproine (NC) in 96% ethanol.
4.15. Mathematical Modelling of Redox-Modulating Capacities
4.16. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, S.; Wen, Z.; Liao, W.; Liu, C.; Kincaid, R.L.; Harrison, J.H.; Elliott, D.C.; Brown, M.D.; Stevens, D.J. Studies into using manure in a biorefinery concept. Appl. Biochem. Biotechnol. 2005, 124, 999–1015. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Octave, S.P.; Thomas, D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef]
- Pandey, A.; Höfer, R.; Taherzadeh, M.; Nampoothiri, K.M.; Larroche, C. Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; p. 710. [Google Scholar]
- Yang, S.; Zhang, Y.; Yue, W.; Wang, W.; Wang, Y.-Y.; Yuan, T.-Q.; Sun, R.-C. Valorization of lignin and cellulose in acid-steam-exploded corn stover by a moderate alkaline ethanol post-treatment based on an integrated biorefinery concept. Biotechnol. Biofuels 2016, 9, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Schuelter, A.R.; Kroumov, A.D.; Hinterholz, C.L.; Fiorini, A.; Trigueros, D.E.G.; Vendruscolo, E.G.; Zaharieva, M.M.; Modenes, A.N. Isolation and identification of new microalgae strains with antibacterial activity on food-borne pathogens. Engineering approach to optimize synthesis of desired metabolites. Biochem. Eng. J. 2019, 144, 28–39. [Google Scholar] [CrossRef]
- Kroumov, A.D.; Módenes, A.N.; Trigueros, D.E.G.; Espinoza-Quiñones, F.R.; Borba, C.E.; Scheufele, F.B.; Hinterholz, C.L. A systems approach for CO2 fixation from flue gas by microalgae—Theory review. Process Biochem. 2016, 51, 1817–1832. [Google Scholar] [CrossRef]
- Kroumov, A.D.; Scheufele, F.B.; Trigueros, D.E.G.; Modenes, A.N.; Zaharieva, M.; Najdenski, H. Chapter 11-Modeling and Technoeconomic Analysis of Algae for Bioenergy and Coproducts. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 201–241. [Google Scholar]
- Hinterholz, C.; Schuelter, A.; Módenes, A.; Trigueros, D.; Borba, C.; Espinoza-Quiñones, F.; Kroumov, A. Microalgae Flat Plate-Photobioreactor (FP-PBR) System Development: Computational Tools to Improve Experimental Results. Acta Microbiol. Bulg. 2017, 33, 119–124. [Google Scholar]
- Hinterholz, C.L.; Trigueros, D.E.G.; Módenes, A.N.; Borba, C.E.; Scheufele, F.B.; Schuelter, A.R.; Kroumov, A.D. Computational fluid dynamics applied for the improvement of a flat-plate photobioreactor towards high-density microalgae cultures. Biochem. Eng. J. 2019, 151, 107257. [Google Scholar] [CrossRef]
- Kroumov, A.; Gacheva, G.; Iliev, I.; Alexandrov, S.; Pilarski, P.; Petkov, G. Analysis of Sf/V ratio of photobioreactors linked with algal physiology. Genet. Plant Physiol. 2013, 3, 55–64. [Google Scholar]
- Gonçalves, V.D.; Fagundes-Klen, M.R.; Trigueros, D.E.G.; Schuelter, A.R.; Kroumov, A.D.; Módenes, A.N. Combination of Light Emitting Diodes (LEDs) for photostimulation of carotenoids and chlorophylls synthesis in Tetradesmus sp. Algal Res. 2019, 43, 101649. [Google Scholar] [CrossRef]
- Aburai, N.; Sumida, D.; Abe, K. Effect of light level and salinity on the composition and accumulation of free and ester-type carotenoids in the aerial microalga Scenedesmus sp. (Chlorophyceae). Algal Res. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Humbeck, K. Light-dependent carotenoid biosynthesis in mutant C-6D of Scenedesmus obliquus. Photochem. Photobiol. 1990, 51, 113–118. [Google Scholar] [CrossRef]
- Kona, R.; Pallerla, P.; Addipilli, R.; Sripadi, P.; Venkata Mohan, S. Lutein and ß-carotene biosynthesis in Scenedesmus sp. SVMIICT1 through differential light intensities. Bioresour. Technol. 2021, 341, 125814. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, R.; Mallick, N. Utilization of Scenedesmus obliquus biomass as feedstock for biodiesel and other industrially important co-products: An integrated paradigm for microalgal biorefinery. Algal Res. 2015, 12, 328–336. [Google Scholar] [CrossRef]
- Qin, S.; Liu, G.-X.; Hu, Z.-Y. The accumulation and metabolism of astaxanthin in Scenedesmus obliquus (Chlorophyceae). Process. Biochem. 2008, 43, 795–802. [Google Scholar] [CrossRef]
- Swiatkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. Application of microalgae biomass in poultry nutrition. World’s Poult. Sci. J. 2015, 71, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Bonos, E.; Kasapidou, E.; Kargopoulos, A.; Karampampas, A.; Christaki, E.; Florou-Paneri, P.; Nikolakakis, I. Spirulina as a functional ingredient in broiler chicken diets. S. Afr. J. Anim. Sci. 2016, 46, 94. [Google Scholar] [CrossRef] [Green Version]
- El-Abd, N.M.; Hamouda, R.A.E.-F. Improved Productivity and Health of Broiler Chicken by Micro Green Alga Chlorella vulgaris. Asian J. Poult. Sci. 2017, 11, 57–63. [Google Scholar]
- Evans, A.M.; Smith, D.L.; Moritz, J.S. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 d bird performance. J. Appl. Poult. Res. 2015, 24, 206–214. [Google Scholar] [CrossRef]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- El-Baz, F.; Abdo, S.; El-Sayed, D.; Mostafa, M.; Elsherif, H.; Safaa, H.; Abdon, A. Application of Defatted Scenedesmus Obliquus Biomass for Broilers’ Nutrition. Braz. J. Poult. Sci. 2021, 23. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals; Elsevier Inc.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Austic, R.; Mustafa, A.; Jung, B.; Gatrell, S.; Lei, X. Potential and Limitation of a New Defatted Diatom Microalgal Biomass in Replacing Soybean Meal and Corn in Diets for Broiler Chickens. J. Agric. Food Chem. 2021, 61, 7341–7348. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Shankara Murthy, V.; Chauhan, V.; Peddha, M.; Sarada, R. Characterisation of defatted Scenedesmus dimorphus algal biomass as animal feed. J. Appl. Phycol. 2014, 27, 1871–1879. [Google Scholar] [CrossRef]
- Elfata, R.A.; Abou, G.W. Influence of Various Concentrations of Phosphorus on the Antibacterial, Antioxidant and Bioactive Components of Green Microalgae Scenedesmus obliquus. Int. J. Pharmacol. 2017, 14, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Marrez, D.A.; Naguib, M.M.; Sultan, Y.Y.; Higazy, A.M. Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon 2019, 5, e01404. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhang, L.; Zhao, Z.-Y.; Ma, S.-S.; Wang, M.; Liu, P.-H. Prescreening, identification and harvesting of microalgae with antibacterial activity. Biologia 2016, 71, 1111–1118. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Mudimu, O.; Rybalka, N.; Bauersachs, T.; Born, J.; Friedl, T.; Schulz, R. Biotechnological screening of microalgal and cyanobacterial strains for biogas production and antibacterial and antifungal effects. Metabolites 2014, 4, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Liebezeit, G. Screening for biological activities and toxicological effects of 63 phytoplankton species isolated from freshwater, marine and brackish water habitats. Harmful Algae 2012, 20, 58–70. [Google Scholar] [CrossRef]
- Selivanova, E.A.; Ignatenko, M.E.; Nemtseva, N.V. Antagonistic activity of novel green microalgae strain. Zhurnal Mikrobiol. Epidemiol. i Immunobiol. 2014, 4, 72–76. [Google Scholar]
- Catarina Guedes, A.; Barbosa, C.R.; Amaro, H.M.; Pereira, C.I.; Xavier Malcata, F. Microalgal and cyanobacterial cell extracts for use as natural antibacterial additives against food pathogens. Int. J. Food Sci. Technol. 2011, 46, 862–870. [Google Scholar] [CrossRef]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. In Seminars in Cancer Biology; Academic Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011, 64, 325–337. [Google Scholar]
- Fayaz, M.; Namitha, K.K.; Murthy, K.N.; Swamy, M.M.; Sarada, R.; Khanam, S.; Subbarao, P.V.; Ravishankar, G.A. Chemical composition, iron bioavailability, and antioxidant activity of Kappaphycus alvarezzi (Doty). J. Agric. Food Chem. 2005, 53, 792–797. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Sansone, C.; Brunet, C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Rep. 2016, 10, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.Q.; Wang, L.R.; Zhang, Z.X.; Sun, X.M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [Green Version]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [Green Version]
- de Morais, M.G.; Vaz, B.d.S.; de Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Z.; Imanpour Namin, J.; Ramezanpour, Z. Evaluation of antimicrobial activities of microalgae Scenedesmus dimorphus extracts against bacterial strains. Caspian J. Environ. Sci. 2018, 16, 25–36. [Google Scholar]
- Sayegh, F.; Elazzazy, A.; Bellou, S.; Moustogianni, A.; Elkady, A.I.; Baeshen, M.N.; Aggelis, G. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann. Microbiol. 2016, 66, 937–948. [Google Scholar] [CrossRef]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.E.; Rothrock, M.J.; Mishra, A. Mapping foodborne pathogen contamination throughout the conventional and alternative poultry supply chains. Poult. Sci. 2021, 100, 101157. [Google Scholar] [CrossRef] [PubMed]
- Fancher, C.A.; Zhang, L.; Kiess, A.S.; Adhikari, P.A.; Dinh, T.T.N.; Sukumaran, A.T. Avian Pathogenic Escherichia coli and Clostridium perfringens: Challenges in No Antibiotics Ever Broiler Production and Potential Solutions. Microorganisms 2020, 8, 1533. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 276. [Google Scholar]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance; IntechOpen: London, UK, 2018. [Google Scholar]
- Roth, N.; Kä¤sbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Boskovic, M.; Djordjevic, J.; Glisic, M.; Ciric, J.; Janjic, J.; Zdravkovic, N.; Krnjaic, D.; Baltic, M.Z. The effect of oregano (Origanum vulgare) essential oil on four Salmonella serovars and shelf life of refrigerated pork meat packaged under vacuum and modified atmosphere. J. Food Processing Preserv. 2020, 44, e14311. [Google Scholar] [CrossRef]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial Activity of Thyme (Tymus vulgaris) and Oregano (Origanum vulgare) Essential Oils against Some Food-borne Microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Cattelan, M.; Castilhos, M.; Sales, P.; Hoffmann, F. Antibacterial activity of oregano essential oil against foodborne pathogens. Nutr. Food Sci. 2013, 43, 954–965. [Google Scholar]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the Antimicrobial Activity of Oregano Oil by Encapsulation in Chitosan—Alginate Nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Palou, E.; Lopez-Malo, A.; Barros-Velazquez, J. Chapter 47-Combinational Approaches for Antimicrobial Packaging: Chitosan and Oregano Oil. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 581–588. [Google Scholar]
- Carrion-Granda, X.; Fernandez-Pan, I.; Jaime, I.; Rovira, J.; Mate, J.I. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int. J. Food Microbiol. 2016, 232, 144–149. [Google Scholar] [CrossRef]
- Fuzette Amaral, D.M.; Bhargava, K. Essential Oil Nanoemulsions and Food Applications. Adv. Food Technol. Nutr. Sci.-Open J. 2015, 1, 84–87. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Lambrianidi, L.; Savvaidis, I.N.; Tsiraki, M.I.; El-Obeid, T. Chitosan and Oregano Oil Treatments, Individually or in Combination, Used to Increase the Shelf Life of Vacuum-Packaged, Refrigerated European Eel (Anguilla anguilla) Fillets. J. Food Prot. 2019, 82, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, L.; Hume, M.; Rodriguez, F.; Nisbet, D.; Sohail, M.U.; Afanador-Tellez, G. Effects of Colombian oregano essential oil (Lippia origanoides Kunth) and Eimeria species on broiler production and cecal microbiota. Poult. Sci. 2019, 98, 4777–4786. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Q.Y.; Liu, Y.R.; Ma, Q.G.; Zhang, J.Y.; Guo, Y.P.; Xue, Z.; Zhao, L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021, 100, 101163. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Shaheen, H.M.; Abdel-Latif, M.A.; Noreldin, A.E.; Patra, A.K. The usefulness of oregano and its derivatives in poultry nutrition. World’s Poult. Sci. J. 2018, 74, 463–474. [Google Scholar] [CrossRef]
- Abouelezz, K.; Abou-Hadied, M.; Yuan, J.; Elokil, A.A.; Wang, G.; Wang, S.; Wang, J.; Bian, G. Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal 2019, 13, 2216–2222. [Google Scholar] [CrossRef]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.H.; Gottardo, C.; Suntres, Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum vulgare Extract and Its Major Constituents. Oxid Med. Cell Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef] [Green Version]
- Kosakowska, O.; Weglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Krasniewska, K.; Gniewosz, M.; Baczek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Kroumov, A.D.; Módenes, A.N.; Trigueros, D.E.G. A Complex Theoretical Approach for Algal Medium Optimization for CO2 Fixation from Flue Gas. Acta Microbiol. Bulg. 2015, 31, 61–70. [Google Scholar]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef] [Green Version]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Liang, Y.; Yan, G.Y.; Wu, J.L.; Zong, X.; Liu, Z.; Zhou, H.; Liu, L.; Li, N. Qualitative and quantitative analysis of lipo-alkaloids and fatty acids in Aconitum carmichaelii using LC-MS and GC-MS. Phytochem. Anal. 2018, 29, 398–405. [Google Scholar] [CrossRef]
- Ilieva, Y.; Dimitrova, L.; Zaharieva, M.M.; Kaleva, M.; Alov, P.; Tsakovska, I.; Pencheva, T.; Pencheva-El Tibi, I.; Najdenski, H.; Pajeva, I. Cytotoxicity and Microbicidal Activity of Commonly Used Organic Solvents: A Comparative Study and Application to a Standardized Extract from Vaccinium macrocarpon. Toxics 2021, 9, 92. [Google Scholar] [CrossRef]
- EUCAST. Clinical Breakpoints-Breakpoints and Guidance. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 28 November 2021).
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Cokol-Cakmak, M.; Cokol, M. Miniaturized Checkerboard Assays to Measure Antibiotic Interactions. Methods Mol. Biol. 2019, 1939, 3–9. [Google Scholar]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/36406.html (accessed on 29 November 2021).
- Zuorro, A.; Lavecchia, R.; Maffei, G.; Marra, F.; Miglietta, S.; Petrangeli, A.; Familiari, G.; Valente, T. Enhanced Lipid Extraction from Unbroken Microalgal Cells Using Enzymes. Chem. Eng. Trans. 2015, 43, 211–216. [Google Scholar]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of cell wall degrading enzymes to improve the recovery of lipids from Chlorella sorokiniana. Chem. Eng. J. 2019, 377, 120325. [Google Scholar] [CrossRef]
- Scheufele, F.B.; Hinterholz, C.L.; Zaharieva, M.M.; Najdenski, H.M.; Modenes, A.N.; Trigueros, D.E.G.; Borba, C.E.; Espinoza-Quinones, F.R.; Kroumov, A.D. Complex mathematical analysis of photobioreactor system. Eng. Life Sci. 2018, 19, 844–859. [Google Scholar] [CrossRef] [Green Version]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen Gupta, S.; Ghosh, M. In Vitro Antioxidative Evaluation of α- and β-Carotene, Isolated from Crude Palm Oil. J. Anal. Methods Chem. 2013, 2013, 351671. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, H.S.; Ibrahim, H.A.H.; Beltagy, E.A.; Khairy, H.M. Effects of short term feeding of some marine microalgae on the microbial profile associated with Dicentrarchus labrax post larvae. Egypt. J. Aquat. Res. 2014, 40, 251–260. [Google Scholar] [CrossRef] [Green Version]
- González-Davis, O.; Ponce-Rivas, E.; Sánchez-Saavedra, M.D.P.; Muñoz-Márquez, M.-E.; Gerwick, W.H. Bioprospection of Microalgae and Cyanobacteria as Biocontrol Agents Against Vibrio campbellii and Their Use in White Shrimp Litopenaeus vannamei Culture. J. World Aquac. Soc. 2012, 43, 387–399. [Google Scholar] [CrossRef]
- Kokou, F.; Makridis, P.; Kentouri, M.; Divanach, P. Antibacterial activity in microalgae cultures. Aquac. Res. 2012, 43, 1520–1527. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; Reina, G.; Señoráns, F.; Ibáñez, E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT-Food Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Pratita, A.T.K.; Fathurohman, M.; Ruswanto, R.; Khusnul; Suhartati, R. Potential of Autotroph Microalgae (Spirulina plantentis) as Antimicrobial agent. J. Phys. Conf. Ser. 2019, 1179, 012173. [Google Scholar] [CrossRef] [Green Version]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Kim, K.-R.; Oh, D.-K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Gutiérrez-Del-Río, I.; López, Y.; Redondo-Blanco, S.; Gabasa, Y.; Iglesias, M.J.; Soengas, R.; Fernández-Lorenzo, A.; López-Ibáñez, S.; Villar, C.J.; et al. Microalgae and Cyanobacteria Strains as Producers of Lipids with Antibacterial and Antibiofilm Activity. Mar. Drugs 2021, 19, 675. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Ambati, R.R.; Reddy, A.H.; Aradhya, S.M. Antibacterial properties of Spirulina platensis, Haematococcus pluvialis, Botryococcus braunii micro algal extracts. Curr. Trends Biotechnol. Pharm. 2010, 4, 809–819. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Baskan, K.S.; Tütem, E.; Ozer, N.; Apak, R. Spectrophotometric and Chromatographic Assessment of Contributions of Carotenoids and Chlorophylls to the Total Antioxidant Capacities of Plant Foods. J. Agric. Food Chem. 2013, 61, 11371–11381. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids-Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gıdık, B. Antioxidant, Antimicrobial Activities and Fatty Acid Compositions of Wild Berberis spp. by Different Techniques Combined with Chemometrics (PCA and HCA). Molecules 2021, 26, 7448. [Google Scholar] [CrossRef] [PubMed]
- Mohadjerani, M.; Tavakoli, R.; Hosseinzadeh, R. Fatty acid composition, antioxidant and antibacterial activities of Adonis wolgensis L. extract. Avicenna J. Phytomed. 2014, 4, 24–30. [Google Scholar]
- Mandalam, R.K.; Palsson, B.O. Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol. Bioeng. 1998, 59, 605–611. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopoeia. European Pharmacopoeia 8th edition, a. Tannins in herbal drugs. European Directorate for the Quality of Medicines & HealthCare (EDQM). Eur. Pharm. 2013, 275–276. [Google Scholar]
- European Pharmacopoeia. European Pharmacopoeia 8th edition, b. Safflower flower. European Directorate for the Quality of Medicines & HealthCare (EDQM). Eur. Pharm. 2013, 1371–1372. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Carpio, R.; de Leon, R.; Martinez-Goss, M. Growth, lipid content, and lipid profile of the green alga, Chlorella vulgaris Beij. under different concentrations of Fe and CO2. J. Eng. Sci. Technol. 2015, 10, 19–30. [Google Scholar]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Yıldıztugay, E.; Zheleva-Dimitrova, D.; Picot-Allain, C.; Mahomoodally, M.F.; Imran, M.; Dall’Acqua, S. UHPLC-MS Characterization and Biological Insights of Different Solvent Extracts of Two Achillea Species (A. aleppica and A. santolinoides) from Turkey. Antioxidants 2021, 10, 1180. [Google Scholar] [CrossRef]
- ISO20776/1-2006; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. Technical Committee CEN/TC 140, Technical Committee ISO/TC 21. European Committee for Standardization (CEN): Brussels, Belgium, 2006; p. 19.
- Breakpoint Tables for Bacteria. Available online: http://www.eucast.org/clinical_breakpoints/Clinical breakpoints (accessed on 6 January 2021).
- Jenkins, S.G.; Schuetz, A.N. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 2012, 87, 290–308. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cheng, H.; Wang, F.; Wei, D.; Wang, X. An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J. Microbiol. Methods 2010, 82, 330–333. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.M.; Trochopoulos, A.; Dimitrova, L.; Berger, M.R.; Najdenski, H.; Konstantinov, S.; Kroumov, A.D. New Insights in Routine Procedure for Mathematical Evaluation of in vitro Cytotoxicity Data from Cancer Cell Lines. Int. J. Bioautom. 2018, 22, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, A.; Ilieva, Y.; Kokanova-Nedialkova, Z.; Zaharieva, M.M.; Nedialkov, P.; Dobreva, A.; Kroumov, A.; Najdenski, H.; Mileva, M. Redox-Modulating Capacity and Antineoplastic Activity of Wastewater Obtained from the Distillation of the Essential Oils of Four Bulgarian Oil-Bearing Roses. Antioxidants 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]



| Cultivation Time [Days] | Biomass Concentration—Dry Weight [g/L] | |
|---|---|---|
| SC-PBR1 (Green Light) | SC-PBR2 (Red Light) | |
| 0 | 0.32 | 0.41 |
| 6 | 2.85 | 2.18 |
| 14 | 3.36 | 2.61 |
| 21 | 4.32 | 4.78 |
| 29 | 6.95 | 5.87 |
| 36 | 5.72 | 5.55 |
| Microalgal Strain | Flavonoids % (g/100 g dw) | Polyphenols % (g/100 g dw) | Chlorophyll a mg/g dw | Chlorophyll b mg/g dw | Total Carotenoids mg/g dw | Lipids % (g/100 g dw) |
|---|---|---|---|---|---|---|
| S. obliquus, green light, SC-PBR1 | 0.85 ± 0.11 | 1.49 ± 0.15 | 16.38 ± 0.44 | 6.73 ± 0.21 | 5.49 ± 0.18 | 8.88 ± 0.12 |
| S. obliquus, red light, SC-PBR2 | 0.61 ± 0.09 | 2.15 ± 0.07 | 16.80 ± 0.28 | 7.88 ± 0.29 | 5.14 ± 0.15 | 8.97 ± 0.13 |
| Statistical analysis (p-value) * | 0.0353 | 0.0021 | 0.2382 | 0.0053 | 0.0671 | 0.4541 |
| № | Tentatively Annotated Compound | Molecular Formula | Exact Mass [M + H]+ | Fragmentation Pattern (Relative Abundance) | tR (min) |
|---|---|---|---|---|---|
| 1 | carotene | C40H56 | 537.4455 | 537.4359 (100), 406.3410 (9.3), 322.2479 (3.4), 198.1847 (9.9), 114.0914 (38.6), 95.0858 (1.6), 69.0705 (10.3) | 11.26 |
| 2 | canthaxanthin | C40H52O2 | 565.4040 | 565.4030 | 22.60 |
| 3 | hydroxyechineone | C40H54O2 | 567.4197 | 567.4184 (100), 549.4068 (11.9), 169.1007 (20.9), 147.1163 (8.8), 145.1008 (45.0), 119.0857 (60.5), 105.0701 (83.24), 93.0704 (47.5), 69.0705 (8.3) | 22.90 |
| 4 | lutein/zeaxanthin | C40H56O2 | 569.4353 | 569.4304 | 22.70 |
| 5 | neoxanthin/violaxanthin | C40H56O4 | 601.4251 | 601.4150 | 22.62 |
| Tentatively Annotated Compound | Molecular Formula | Exact Mass [M − H]− | Fragmentation Pattern (Relative Abundance) | tR (min) | |
| 6 | hydroxyhexadecatetraenoic acid | C16H24O3 | 263.1658 | 263.1656 (81.22), 245.1541 (23.47), 242.9861 (27.09), 219.1750 (13.63), 205.1228 (36.81), 201.1643 (100), 173.1328 (5.00), 161.1325 (67.24), 159.165 (3.61), 147.1169 (25.76), 133.1002 (3.25), 107.0853 (23.33), 97.0643 (4.17), 71.0486 (39.93), 59.0123 (2.58), 57.0329 (12.89) | 13.15 |
| 7 | hydroxyhexadecatrienoic acid | C16H26O3 | 265.1811 | 265.1811 (100), 247.1703 (70.26), 229.1596 (0.80), 207.1385 (94.60), 181.1222 (0.97), 163.1480 (5.12), 149.1324 (3.25), 83.0485 (1.10), 71.0487 (9.99), 59.0123 (12.30), 57.0330 (1.31) | 13.78 |
| 8 | hydroxyhexadecadienoic acid | C16H28O3 | 267.1968 | 267.1968 (100), 249.1862 (34.79), 216.9886 (1.37), 205.1955 (1.13), 167.1068 (54.06), 149.0961 (3.35), 59.0123 (7.73) | 15.04 |
| 9 | octadecatetraenoic acid (stearidonic acid) | C18H28O2 | 275.2015 | 275.2015 (100), 231.2119 (4.30), 177.1634 (2.06), 59.0123 (4.58) | 15.94 |
| 10 | dihydroxyhexadecapentaenoic acid | C16H22O4 | 277.1451 | 277.1447 (79.51), 259.1348 (5.20), 249.2476 (1.29), 233.1526 (3.73), 221.1182 (41.22), 177.1275 (63.60), 161.0961 (14.63), 149.0961 (16.73), 135.0802 (100), 121.0646 (20.28), 97.0644 (64.25), 95.0487 (13.82), 71.0487 (31.35), 59.0123 (21.09), 57.0330 (9.94) | |
| 11 | dihydroxyhexadecatetraenoic acid | C16H24O4 | 279.1609 | 279.1595 (19.30), 261.1496 (13.77), 207.1021 (85.26), 181.0863 (29.95), 163.1113 (2.64), 157.0860 (65.28), 139.0750 (6.96), 121.0645 (51.56), 97.0644 (100), 95.0488 (10.09), 83.0487 (10.53), 65.0381 (35.25), 59.0123 (7.19) | 10.24 |
| 12 | hydroxyoctadecatrienoic acid (hydroxylinolenic acid) | C18H30O3 | 293.2127 | 293.2126 (80.24), 275.2017 (100), 231.2111 (5.27), 183.1019 (0.87), 171.1018 (3.33), 121.1008 (1.76), 71.0486 (2.13) | 15.92 |
| 13 | hydroxyoctadecatrienoic acid isomer | C18H30O3 | 293.2125 | 293.2125 (100), 275.2021 (8.55), 231.2133 (0.48), 223.1336 (14.32), 195.1383 (12.33), 179.1431 (0.79), 111.0799 (0.55), 87.0948 (0.48), 71.0035 (0.60), 59.0121 (0.58) | 16.12 |
| 14 | dihydroxyoctadecapentaenoic acid | C18H26O4 | 305.1767 | 305.1763 (100), 287.1656 (9.13), 269.1551 (1.59), 233.1180 (1.94), 221.1179 (2.00), 205.1594 (5.28), 185.1177 (2.68), 163.1124 (1.62), 151.1119 (1.92), 135.0803 (98.45), 125.0959 (19.2), 97.0644 (16.61), 79.0538 (11.99), 57.0330 (2.23) | 13.25 |
| 15 | dihydroxyoctadecatetraenoic acid | C18H28O4 | 307.1923 | 307.1921 (37.36), 289.1814 (23.68), 235.1338 (100), 211.1335 (38.20), 185.1175 (87.44), 167.1442 (0.73), 141.1270 (1.03), 137.0961 (2.41), 125.0959 (33.31), 121.0645 (91.93), 97.0644 (64.24), 83.0488 (0.99), 65.0381 (42.23), 71.0487 (32.56), 57.0329 (2.65) | 12.30 |
| 16 | dihydroxyoctadecatrienoic acid | C18H30O4 | 309.2080 | 309.2076 (100), 291.1970 (59.85), 273.1873 (8.87), 229.1957 (4.12), 263.2017 (4.89), 251.1652 (56.36), 225.1493 (37.13), 209.1541 (83.09), 197.1175 (41.33), 175.1483 (1.44), 135.1164 (1.17), 11,100,799 (14.94), 97.0641 (5.35), 83.0487 (2.08), 71.0486 (7.65), 57.0331 (0.96) | 14.03 |
| 17 | dihydroxyoctadecadienoic acid | C18H32O4 | 311.2237 | 311.2232 (100), 293.2125 (15.19),275.2009 (2.93), 249.2224 (0.56), 227.2135 (0.28), 211.1335 (15.79), 197.1177 (8.26), 171.1017 (17.25), 139.1116 (3.32), 129.0907 (7.02), 113.0956 (2.08), 99.0798 (1.62), 83.0488 (0.42), 57.0330 (1.32) | 15.11 |
| 18 | trihydroxyoctadecatetraenoic acid | C18H28O5 | 323.1873 | 323.1861 (61.84), 305.1762 (68.47), 287.1656 (58.74), 243.1755 (9.49), 237.1495 (89.71), 209.1178 (56.60), 171.1013 (14.89), 151.0754 (10.46), 135.0801 (11.94), 125.0958 (11.83), 113.0594 (100), 95.0487 (57.09), 83.0488 (26.95), 71.0487 (28.29), 57.0332 (12.39) | 12.40 |
| 19 | trihydroxyoctadecatrienoic acid | C18H30O5 | 325.2017 | 325.2017 (24.32), 307.1913 (19.90), 289.1813 (83.61), 245.1910 (6.28), 237.1495 (100), 211.1335 (1.82), 201.1126 (49.71), 197.1170 (2.71), 171.1021 (2.09), 123.0804 (3.10), 109.0646 (6.46), 83.0486 (3.38), 57.0331 (9.74) | 11.29 |
| 20 | trihydroxyoctadecenoic acid | C18H34O5 | 329.2342 | 329.2337 (100), 311.2227 (0.90), 293.2119 (0.92), 268.9841 (0.37), 229.1442 (6.79), 211.1337 (10.37), 183.1380 (0.92), 171.1017 (26.15), 157.1231 (1.46), 139.1116 (9.08), 127.1115 (3.29), 99.0801 (4.46), 69.0964 (0.89), 87.0329 (1.24) | 10.68 |
| Extracts of Lyophilized Biomass from: | S. aureus | E. coli | S. typhimurium | |||
|---|---|---|---|---|---|---|
| MIC [mg/mL] | DEHA [%] | MIC [mg/mL] | DEHA [%] | MIC [mg/mL] | DEHA [%] | |
| S. obliquus, green light (E1) | >12.5 | - | 12.5 | 16 ± 0.31 | 12.5 | 5.2 ± 0.7 |
| S. obliquus, red light (E2) | >12.5 | - | 12.5 | 10.4 ± 0.28 | 12.5 | 1.09 ± 0.14 |
| Bacterial Species | Minimal Inhibitory Concentrations | |||
|---|---|---|---|---|
| [mg/L] | [%] | |||
| PEN * | CIP ** | ENR *** | OrO **** | |
| S. aureus | 0.125 | 0.25 | 0.05 | 0.05 § |
| E. coli | - | 0.0125 | 0.0125 | 0.05 § |
| S. typhimurium | - | 0.05 | 0.05 | 0.05 |
| Strain | AB/CT/OrO | Extract | MICC-extract | MICC-AB/CT/OrO | FICextract | FICAB/CT/OrO | ∑FIC | Effect |
|---|---|---|---|---|---|---|---|---|
| S. aureus | PEN | E1 | 0.01 | 0.0625 | 0.0008 | 0.5 | 0.5008 | Additive |
| E2 | 0.01 | 0.0625 | 0.0008 | 0.5 | 0.5008 | Additive | ||
| CIP | E1 | 0.01 | 0.0625 | 0.0008 | 0.25 | 0.2508 | Synergism | |
| E2 | 0.01 | 0.0625 | 0.0008 | 0.25 | 0.2508 | Synergism | ||
| ENR | E1 | 0.01 | 0.05 | 0.0008 | 1 | 1.0008 | Indifference | |
| E2 | 0.01 | 0.05 | 0.0008 | 1 | 1.0008 | Indifference | ||
| OrO | E1 | 0.005 | 0.025 | 0.0004 | 0.5 | 0.5008 | Additive | |
| 0.025 | 0.0125 | 0.002 | 0.25 | 0.252 | Synergism | |||
| E2 | 0.01 | 0.025 | 0.0008 | 0.5 | 0.5008 | Additive | ||
| E. coli | CIP | E1 | 0.01 | 0.003125 | 0.0008 | 0.5 | 0.5008 | Synergism |
| E2 | 0.01 | 0.003125 | 0.0008 | 0.5 | 0.5008 | Synergism | ||
| ENR | E1 | 0.01 | 0.00625 | 0.0008 | 0.5 | 0.5008 | Additive | |
| E2 | 0.01 | 0.00625 | 0.0008 | 0.5 | 0.5008 | Additive | ||
| OrO | E1 | 0.005 | 0.025 | 0.0004 | 0.5 | 0.5004 | Additive | |
| 0.025 | 0.0125 | 0.002 | 0.25 | 0.252 | Synergism | |||
| E2 | 0.005 | 0.025 | 0.0004 | 0.5 | 0.5004 | Additive | ||
| 0.01 | 0.0125 | 0.0008 | 0.25 | 0.5008 | Synergism | |||
| S. typhimurium | CIP | E1 | 0.01 | 0.0125 | 0.0008 | 0.25 | 0.2508 | Synergism |
| E2 | 0.01 | 0.0125 | 0.0008 | 0.25 | 0.2508 | Synergism | ||
| ENR | E1 | 0.01 | 0.025 | 0.0008 | 0.5 | 0.5008 | Additive | |
| E2 | 0.01 | 0.025 | 0.0008 | 0.5 | 0.5008 | Additive | ||
| OrO | E1 | 0.005 | 0.025 | 0.0004 | 0.5 | 0.5004 | Additive | |
| 0.025 | 0.0125 | 0.002 | 0.25 | 0.252 | Synergism | |||
| E2 | 0.01 | 0.0125 | 0.0008 | 0.25 | 0.2508 | Synergism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, M.M.; Zheleva-Dimitrova, D.; Rusinova-Videva, S.; Ilieva, Y.; Brachkova, A.; Balabanova, V.; Gevrenova, R.; Kim, T.C.; Kaleva, M.; Georgieva, A.; et al. Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept. Molecules 2022, 27, 519. https://doi.org/10.3390/molecules27020519
Zaharieva MM, Zheleva-Dimitrova D, Rusinova-Videva S, Ilieva Y, Brachkova A, Balabanova V, Gevrenova R, Kim TC, Kaleva M, Georgieva A, et al. Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept. Molecules. 2022; 27(2):519. https://doi.org/10.3390/molecules27020519
Chicago/Turabian StyleZaharieva, Maya Margaritova, Dimitrina Zheleva-Dimitrova, Snezhana Rusinova-Videva, Yana Ilieva, Anna Brachkova, Vessela Balabanova, Reneta Gevrenova, Tanya Chan Kim, Mila Kaleva, Almira Georgieva, and et al. 2022. "Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept" Molecules 27, no. 2: 519. https://doi.org/10.3390/molecules27020519