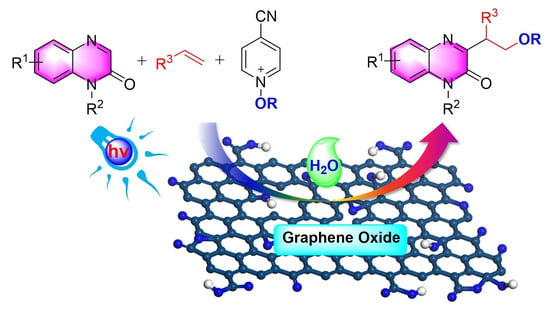
Photoinitiated Multicomponent Anti-Markovnikov Alkoxylation over Graphene Oxide
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure of the Products 4
3.3. Characterization Data of Products 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Bielawski, C.W. Carbocatalysis: Heterogeneous carbons finding utility in synthetic chemistry. Chem. Sci. 2011, 2, 1233–1240. [Google Scholar] [CrossRef]
- Su, C.; Loh, K.P. Carbocatalysts: Graphene oxide and its derivatives. Acc. Chem. Res. 2013, 46, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Carbocatalysis: The state of “metal-free” catalysis. Chem. Eur. J. 2015, 21, 12550–12562. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.-P.; Todd, A.D.; Geng, J.; Bielawski, C.W. Graphite oxide: A selective and highly efficient oxidant of thiols and sulfides. Org. Biomol. Chem. 2011, 9, 7292–7295. [Google Scholar] [CrossRef]
- Shaikh, M.; Sahu, A.; Kumar, A.K.; Sahu, M.; Singh, S.K.; Ranganath, K.V.S. Metal-free carbon as a catalyst for oxidative coupling: Solvent-enhanced poly-coupling with regioselectivity. Green Chem. 2017, 19, 4533–4537. [Google Scholar] [CrossRef]
- Peng, X.-J.; Xu, X.-Y.; Liu, Q.; Liu, L.-X. Graphene oxide and its derivatives: Their synthesis and use in organic synthesis. Curr. Org. Chem. 2019, 23, 188–204. [Google Scholar] [CrossRef]
- Bahuguna, A.; Kumar, A.; Krishnan, V. Carbon-support-based heterogeneous nanocatalysts: Synthesis and applications in organic reactions. Asian J. Org. Chem. 2019, 8, 1263–1305. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S.; Kee, C.W.; Dubuisson, E.; Yang, Y.; Loh, K.P.; Tan, C.-H. Graphene oxide and rose bengal: Oxidative C–H functionalisation of tertiary amines using visible light. Green Chem. 2011, 13, 3341–3344. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Teng, C.-Y.; Chen, S.-J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Teng, C.-Y.; Chen, L.-C.; Chen, S.-J.; Teng, H. Graphene oxide-based nanomaterials for efficient photoenergy conversion. J. Mater. Chem. A 2016, 4, 2014–2048. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, F.; Hu, C.; Chen, Y. Generation of alkoxyl radicals by photoredox catalysis enables selective C(sp3)-H functionalization under mild reaction conditions. Angew. Chem. Int. Ed. 2016, 55, 1872–1875. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xu, R.; Chen, Y. Donor-acceptor complex enables alkoxyl radical generation for metal-free C(sp3)-C(sp3) cleavage and allylation/alkenylation. Angew. Chem. Int. Ed. 2017, 56, 12619–12623. [Google Scholar] [CrossRef]
- Guan, H.; Sun, S.; Mao, Y.; Chen, L.; Lu, R.; Huang, J.; Liu, L. Iron(II)-catalyzed site-selective functionalization of unactivated C(sp3)-H bonds guided by alkoxyl radicals. Angew. Chem. Int. Ed. 2018, 57, 11413–11417. [Google Scholar] [CrossRef]
- Jia, K.; Chen, Y. Visible-light-induced alkoxyl radical generation for inert chemical bond cleavage/functionalization. Chem. Commun. 2018, 54, 6105–6112. [Google Scholar] [CrossRef] [PubMed]
- Tsui, E.; Wang, H.; Knowles, R.R. Catalytic generation of alkoxy radicals from unfunctionalized alcohols. Chem. Sci. 2020, 11, 11124–11141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Niu, Y.; He, X.; Chen, S.; Liu, S.; Li, Z.; Chen, X.; Zhang, Y.; Lan, Y.; Shen, X. Tuning the reactivity of alkoxyl radicals from 1,5-hydrogen atom transfer to 1,2-silyl transfer. Nat. Commun. 2021, 12, 2131–2140. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Chen, Y. Investigations on 1,2-hydrogen atom transfer reactivity of alkoxyl radicals under visible-light-induced reaction conditions. Synlett 2021, 32, 356–361. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Alkoxy radicals generation: Facile photocatalytic reduction of N-alkoxyazinium or azolium salts. Chem. Commun. 2019, 55, 3029–3032. [Google Scholar] [CrossRef]
- He, F.-S.; Ye, S.; Wu, J. Recent advances in pyridinium salts as radical reservoirs in organic synthesis. ACS Catal. 2019, 9, 8943–8960. [Google Scholar] [CrossRef]
- Inial, A.; Morlet-Savary, F.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S. Visible-light-mediated access to phosphate esters. Org. Lett. 2020, 22, 4404–4407. [Google Scholar] [CrossRef]
- Rössler, S.L.; Jelier, B.J.; Magnier, E.; Dagousset, G.; Carreira, E.M.; Togni, A. Pyridinium salts as redox-active functional group transfer reagents. Angew. Chem. Int. Ed. 2020, 59, 9264–9280. [Google Scholar] [CrossRef]
- Shukla, D.; Adiga, S.P.; Ahearn, W.G.; Dinnocenzo, J.P.; Farid, S. Chain-amplified photochemical fragmentation of N-alkoxypyridinium salts: Proposed reaction of alkoxyl radicals with pyridine bases to give pyridinyl radicals. J. Org. Chem. 2013, 78, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Park, B.; Kang, G.; Kim, J.; Jung, H.; Lee, H.; Baik, M.-H.; Hong, S. Visible-light-induced pyridylation of remote C(sp3)-H bonds by radical translocation of N-alkoxypyridinium salts. Angew. Chem. Int. Ed. 2018, 57, 15517–15522. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wang, Q.; Zhu, J. Remote-C(sp3)-H arylation and vinylation of N-alkoxypyridinium salts to δ-aryl and δ-vinyl alcohols. Chem.-Eur. J. 2019, 25, 11630–11634. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Q.; Zhu, J. Dual photoredox/copper catalysis for the remote C(sp3)−H functionalization of alcohols and alkyl halides by N-alkoxypyridinium salts. Angew. Chem. Int. Ed. 2019, 58, 2139–2143. [Google Scholar] [CrossRef]
- Barthelemy, A.-L.; Tuccio, B.; Magnier, E.; Dagousset, G. Alkoxyl radicals generated under photoredox catalysis: A strategy for anti-Markovnikov alkoxylation reactions. Angew. Chem. Int. Ed. 2018, 57, 13790–13794. [Google Scholar] [CrossRef]
- Barthelemy, A.L.; Tuccio, B.; Magnier, E.; Dagousset, G. Intermolecular trapping of alkoxyl radicals with alkenes: A new route to ether synthesis. Synlett 2019, 30, 1489–1495. [Google Scholar] [CrossRef]
- Su, C.-L.; Tandiana, R.; Balapanuru, J.; Tang, W.; Pareek, K.; Nai, C.T.; Hayashi, T.; Loh, K.-P. Tandem catalysis of amines using porous graphene oxide. J. Am. Chem. Soc. 2015, 137, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Beckwith, A.L.J.; Schiesser, C.H. Regio- and stereo-selectivity of alkenyl radical ring closure: A theoretical study. Tetrahedron 1985, 41, 3925–3941. [Google Scholar] [CrossRef]
- Spellmeyer, D.C.; Houk, K.N. Force-field model for intramolecular radical additions. J. Org. Chem. 1987, 52, 959–974. [Google Scholar] [CrossRef]
- Peng, X.-J.; Zen, Y.; Liu, Q.; Liu, L.-X.; Wang, H.-S. Graphene oxide as a green carbon material for cross-coupling of indoles with ethers via oxidation and the Friedel-Crafts reaction. Org. Chem. Front. 2019, 6, 3615–3619. [Google Scholar] [CrossRef]
- Peng, X.-J.; Hu, D.; Huang, P.-P.; Liao, H.-W.; Zeng, Y.; Liu, Q.; Liu, L.-X. Graphene oxide: A green oxidant-acid bifunctional carbon material for synthesis of functionalized isoindolin-1-ones via formal amide insertion and substitution. Org. Chem. Front. 2020, 7, 1796–1801. [Google Scholar] [CrossRef]







| Entry | MeO• Source | Variation from the Conditions | Yield b (%) |
|---|---|---|---|
| 1 | 3a | none | 70 |
| 2 | 3b | none | 30 |
| 3 | 3c–3e | none | 0 |
| 4 | 3a | 5 mol% Ru(bpy)3Cl2 instead of GO | 39 |
| 5 | 3a | 5 mol% fac−Ir(ppy)3 instead of GO | 37 |
| 6 | 3a | MeOH | 30 |
| 7 | 3a | DMSO | 40 |
| 8 | 3a | dry CH3CN or CH3CN:H2O (1:1 v/v) | 0 |
| 9 | 3a | 50 wt% GO instead of 80 wt% GO | 38 |
| 10 | 3a | 100 wt% GO instead of 80 wt% GO | 69 |
| 11 | 3a | without light or GO | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Peng, X.; He, H.; Hu, J.; Yao, Z.; Zhou, L.; Yang, M.; Li, F.; Huang, Q.; Liu, L. Photoinitiated Multicomponent Anti-Markovnikov Alkoxylation over Graphene Oxide. Molecules 2022, 27, 475. https://doi.org/10.3390/molecules27020475
Nie L, Peng X, He H, Hu J, Yao Z, Zhou L, Yang M, Li F, Huang Q, Liu L. Photoinitiated Multicomponent Anti-Markovnikov Alkoxylation over Graphene Oxide. Molecules. 2022; 27(2):475. https://doi.org/10.3390/molecules27020475
Chicago/Turabian StyleNie, Liang, Xiangjun Peng, Haiping He, Jian Hu, Zhiyang Yao, Linyi Zhou, Ming Yang, Fan Li, Qing Huang, and Liangxian Liu. 2022. "Photoinitiated Multicomponent Anti-Markovnikov Alkoxylation over Graphene Oxide" Molecules 27, no. 2: 475. https://doi.org/10.3390/molecules27020475