Polyphenols for the Treatment of Ischemic Stroke: New Applications and Insights
Abstract
:1. Introduction
2. Pathophysiology of IS
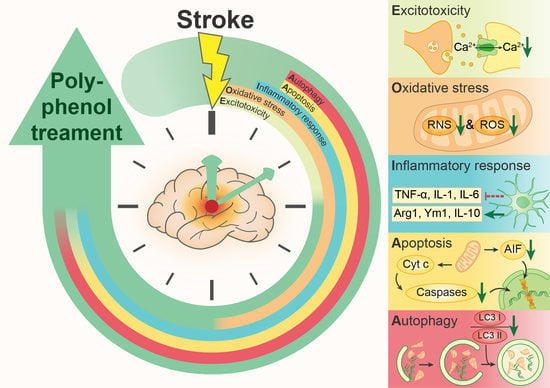
2.1. Excitotoxicity
2.2. Oxidative and Nitrosative Stress
2.3. Inflammatory Response
2.4. Apoptosis
2.5. Autophagy
3. Application of Polyphenols in the Treatment of IS
3.1. Flavonoids
3.1.1. Flavonols
3.1.2. Isoflavones
3.1.3. Flavones
3.1.4. Flavanols
3.1.5. Flavanones
3.1.6. Anthocyanins
3.2. Phenolic Acids
3.2.1. Cinnamic Acid Derivatives
3.2.2. Benzoic Acid Derivatives
3.3. Lignans
3.4. Stilbenes
3.5. Curcumin
3.6. Approaches to Improve the Bioavailability of Polyphenols in IS
3.6.1. Polyphenol Modification Strategies to Improve Bioavailability
3.6.2. Polyphenol Delivery Strategies for IS Therapy
4. Conclusions and Future Prospects
5. Chemical Compounds Studied in This Article
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIF | apoptosis-inducing factor |
| BBB | blood-brain barrier; |
| BDNF | brain-derived neurotrophic factor; |
| CA | chlorogenic acid; |
| CREB | CAMP-response element binding; |
| DAMP | damage-associated molecular pattern; |
| EAAT | excitatory amino acid transporter; |
| ER | endoplasmic reticulum; |
| FA | ferulic acid; |
| FADD | Fas-associated death domain; |
| FasL | first apoptosis signal ligand; |
| GA | gallic acid; IS: ischemic stroke; |
| LIFU | low-intensity focused ultrasound; |
| MIF | migration inhibitory factor; |
| NGF | nerve growth factor; |
| NMDAR | N-methyl-D-aspartate receptor; |
| PA | protocatechuic acid; |
| PRR | pattern recognition receptor; |
| RA | rosmarinic acid; |
| ROS | reactive oxygen species; |
| SAA | salvianolic acid; |
| SD | Sprague Dawley; |
| TLR | toll-like receptor |
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; The GBD 2016 Lifetime Risk of Stroke Collaborators; et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [Green Version]
- Doyle, K.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Bentley, P.; Sharma, P. Pharmacological treatment of ischemic stroke. Pharmacol. Ther. 2005, 108, 334–352. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; MacLeod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischaemic stroke—Renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic Cascades in Ischemic Stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.G.; Chopp, M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol. Sci. 2012, 33, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Cabrer, P.; Campos, F.; Sobrino, T.; Castillo, J. Targeting the Ischemic Penumbra. Stroke 2011, 42, S7–S11. [Google Scholar] [CrossRef] [Green Version]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Lees, K.R.; Bluhmki, E.; von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A.; et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef]
- Hacke, W.; Donnan, G.; Fieschi, C.; Kaste, M.; von Kummer, R.; Broderick, J.P.; Brott, T.; Frankel, M.; Grotta, J.C.; Haley, E.C.; et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004, 363, 768–774. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Schrooten, M.; Bluhmki, E.; Thijs, V.N.; Saver, J. Treatment Time-Specific Number Needed to Treat Estimates for Tissue Plasminogen Activator Therapy in Acute Stroke Based on Shifts over the Entire Range of the Modified Rankin Scale. Stroke 2009, 40, 2079–2084. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.; Saver, J.L. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 2015, 14, 758–767. [Google Scholar] [CrossRef]
- Kaplan-Arabaci, O.; Acari, A.; Ciftci, P.; Gozuacik, D. Glutamate Scavenging as a Neuroreparative Strategy in Ischemic Stroke. Front. Pharmacol. 2022, 13, 1018. [Google Scholar] [CrossRef]
- de Sousa, D.A.; von Martial, R.; Abilleira, S.; Gattringer, T.; Kobayashi, A.; Gallofré, M.; Fazekas, F.; Szikora, I.; Feigin, V.; Caso, V.; et al. Access to and delivery of acute ischaemic stroke treatments: A survey of national scientific societies and stroke experts in 44 European countries. Eur. Stroke J. 2018, 4, 13–28. [Google Scholar] [CrossRef] [Green Version]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.; Horky, L.L.; Van Der Worp, B.H.; Howells, D.W. 1,026 Experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Zhang, S.; Li, J.; Zheng, Y.; Fan, X. The natural (poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke. Eur. J. Pharmacol. 2022, 914, 174660. [Google Scholar] [CrossRef]
- Annunziata, G.; Sureda, A.; Orhan, I.E.; Battino, M.; Arnone, A.; Jiménez-García, M.; Capó, X.; Cabot, J.; Sanadgol, N.; Giampieri, F.; et al. The neuroprotective effects of polyphenols, their role in innate immunity and the interplay with the microbiota. Neurosci. Biobehav. Rev. 2021, 128, 437–453. [Google Scholar] [CrossRef]
- Parrella, E.; Gussago, C.; Porrini, V.; Benarese, M.; Pizzi, M. From Preclinical Stroke Models to Humans: Polyphenols in the Prevention and Treatment of Stroke. Nutrients 2021, 13, 85. [Google Scholar] [CrossRef]
- Pacifici, F.; Rovella, V.; Pastore, D.; Bellia, A.; Abete, P.; Donadel, G.; Santini, S.; Beck, H.; Ricordi, C.; Daniele, N.; et al. Polyphenols and Ischemic Stroke: Insight into One of the Best Strategies for Prevention and Treatment. Nutrients 2021, 13, 1967. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef]
- Shamsi, A.; Anwar, S.; Shahbaaz, M.; Mohammad, T.; Alajmi, M.F.; Hussain, A.; Hassan, I.; Ahmad, F.; Islam, A. Evaluation of Binding of Rosmarinic Acid with Human Transferrin and Its Impact on the Protein Structure: Targeting Polyphenolic Acid-Induced Protection of Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2020, 2020, 1245875. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.; Hasan, P.M.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Jiang, C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta Pharm. Sin. B 2021, 11, 1767–1788. [Google Scholar] [CrossRef]
- Rossi, D.J.; Brady, J.D.; Mohr, C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007, 10, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Obrenovitch, T.P.; Urenjak, J.; Richards, D.A.; Ueda, Y.; Curzon, G.; Symon, L. Extracellular Neuroactive Amino Acids in the Rat Striatum during Ischaemia: Comparison between Penumbral Conditions and Ischaemia with Sustained Anoxic Depolarisation. J. Neurochem. 1993, 61, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pál, B. Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell. Mol. Life Sci. 2018, 75, 2917–2949. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.J.; Esslinger, C.S. The excitatory amino acid transporters: Pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol. Ther. 2005, 107, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001, 24, 107–129. [Google Scholar] [CrossRef]
- Wu, Q.J.; Tymianski, M. Targeting NMDA receptors in stroke: New hope in neuroprotection. Mol. Brain 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Joy, M.T.; Carmichael, S.T. Encouraging an excitable brain state: Mechanisms of brain repair in stroke. Nat. Rev. Neurosci. 2021, 22, 38–53. [Google Scholar] [CrossRef]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007, 182, 1. [Google Scholar] [CrossRef]
- Cabral-Costa, J.V.; Kowaltowski, A.J. Neurological disorders and mitochondria. Mol. Asp. Med. 2020, 71, 100826. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W., 2nd; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- He, Z.; Ning, N.; Zhou, Q.; Khoshnam, S.E.; Farzaneh, M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic. Biol. Med. 2020, 146, 45–58. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Scorziello, A.; Duchen, M. Three Distinct Mechanisms Generate Oxygen Free Radicals in Neurons and Contribute to Cell Death during Anoxia and Reoxygenation. J. Neurosci. 2007, 27, 1129. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–414. [Google Scholar] [CrossRef]
- Parvez, S.; Kaushik, M.; Ali, M.; Alam, M.M.; Ali, J.; Tabassum, H.; Kaushik, P. Dodging blood brain barrier with “nano” warriors: Novel strategy against ischemic stroke. Theranostics 2022, 12, 689–719. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.-C.; Li, Z.-G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Endres, M.; Moro, M.A.; Nolte, C.H.; Dames, C.; Buckwalter, M.S.; Meisel, A. Immune Pathways in Etiology, Acute Phase, and Chronic Sequelae of Ischemic Stroke. Circ. Res. 2022, 130, 1167–1186. [Google Scholar] [CrossRef]
- Dokalis, N.; Prinz, M. Resolution of neuroinflammation: Mechanisms and potential therapeutic option. Semin. Immunopathol. 2019, 41, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.R.; Phan, T.G.; Sobey, C.G. Targeting the Immune System for Ischemic Stroke. Trends Pharmacol. Sci. 2021, 42, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 2020, 21, 2006–2018. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef]
- Mesquida-Veny, F.; Del Río, J.A.; Hervera, A. Macrophagic and microglial complexity after neuronal injury. Prog. Neurobiol. 2021, 200, 101970. [Google Scholar] [CrossRef]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef]
- Xiong, X.-Y.; Liu, L.; Yang, Q.-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.-Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- De Meyer, S.F.; Denorme, F.; Langhauser, F.; Geuss, E.; Fluri, F.; Kleinschnitz, C. Thromboinflammation in Stroke Brain Damage. Stroke 2016, 47, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Del Zoppo, G.J.; Mabuchi, T. Cerebral Microvessel Responses to Focal Ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 879–894. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation—Target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef] [Green Version]
- Neumann, J.; Riek-Burchardt, M.; Herz, J.; Doeppner, T.R.; König, R.; Hütten, H.; Etemire, E.; Männ, L.; Klingberg, A.; Fischer, T.; et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015, 129, 259–277. [Google Scholar] [CrossRef]
- Peña-Martínez, C.; Durán-Laforet, V.; Garcia-Culebras, A.; Ostos, F.; Hernández-Jiménez, M.; Bravo-Ferrer, I.; Pérez-Ruiz, A.; Ballenilla, F.; Díaz-Guzmán, J.; Pradillo, J.; et al. Pharmacological Modulation of Neutrophil Extracellular Traps Reverses Thrombotic Stroke tPA (Tissue-Type Plasminogen Activator) Resistance. Stroke 2019, 50, 3228–3237. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, X.; Gu, L.; Zhu, H.; Zhong, Y.; Ye, Y.; Xiong, X.; Jian, Z. New Insight Into Neutrophils: A Potential Therapeutic Target for Cerebral Ischemia. Front. Immunol. 2021, 12, 692061. [Google Scholar] [CrossRef]
- Splichal, Z.; Jurajda, M.; Duris, K. The Role of Inflammatory Response in Stroke Associated Programmed Cell Death. Curr. Neuropharmacol. 2018, 16, 1365–1374. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Chao, D.T.; Korsmeyer, S.J. BCL-2 FAMILY: Regulators of Cell Death. Annu. Rev. Immunol. 1998, 16, 395–419. [Google Scholar] [CrossRef]
- Alam, M.; Alam, S.; Shamsi, A.; Adnan, M.; Elasbali, A.M.; Abu Al-Soud, W.; Alreshidi, M.; Hawsawi, Y.M.; Tippana, A.; Pasupuleti, V.R.; et al. Bax/Bcl-2 Cascade Is Regulated by the EGFR Pathway: Therapeutic Targeting of Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 869672. [Google Scholar] [CrossRef]
- Yang, J.-L.; Mukda, S.; Chen, S.-D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018, 16, 263–275. [Google Scholar] [CrossRef]
- Kist, M.; Vucic, D. Cell death pathways: Intricate connections and disease implications. EMBO J. 2021, 40, e106700. [Google Scholar] [CrossRef]
- Tuo, Q.-z.; Zhang, S.-t.; Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 2022, 42, 259–305. [Google Scholar] [CrossRef]
- Almeida, A. Genetic determinants of neuronal vulnerability to apoptosis. Cell. Mol. Life Sci. 2013, 70, 71–88. [Google Scholar] [CrossRef]
- Galluzzi, L.; Morselli, E.; Kepp, O.; Kroemer, G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim. Biophys. Acta—Bioenerg. 2009, 1787, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Landshamer, S.; Hoehn, M.; Barth, N.; Duvezin-Caubet, S.; Schwake, G.; Tobaben, S.; Kazhdan, I.; Becattini, B.; Zahler, S.; Vollmar, A.; et al. Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ. 2008, 15, 1553–1563. [Google Scholar] [CrossRef]
- Culmsee, C.; Zhu, C.; Landshamer, S.; Becattini, B.; Wagner, E.; Pellecchia, M.; Blomgren, K.; Plesnila, N. Apoptosis-Inducing Factor Triggered by Poly(ADP-Ribose) Polymerase and Bid Mediates Neuronal Cell Death after Oxygen-Glucose Deprivation and Focal Cerebral Ischemia. J. Neurosci. 2005, 25, 10262. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Nakka, V.P.; Gusain, A.; Mehta, S.L.; Raghubir, R. Molecular Mechanisms of Apoptosis in Cerebral Ischemia: Multiple Neuroprotective Opportunities. Mol. Neurobiol. 2008, 37, 7–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-L.; Li, Y.; Zhao, B.-L.; Li, J.-S.; Zhang, N.; Ye, Z.-H.; Sun, X.-J.; Liu, W.-W. Necroptosis Signaling Pathways in Stroke: From Mechanisms to Therapies. Curr. Neuropharmacol. 2018, 16, 1327–1339. [Google Scholar] [CrossRef]
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G.; Sims, K.; Politei, J.; Banikazemi, M.; Lee, P. Apoptotic Mechanisms after Cerebral Ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Cheng, Q.; Chen, C. The Role of Autophagy in the Pathogenesis of Ischemic Stroke. Curr. Neuropharmacol. 2021, 19, 629–640. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Wang, P.; Shao, B.-Z.; Deng, Z.; Chen, S.; Yue, Z.; Miao, C.-Y. Autophagy in ischemic stroke. Prog. Neurobiol. 2018, 163–164, 98–117. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.-I.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 Association with the ULK1–Atg13–FIP200 Complex Required for Autophagy. Mol. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Jun, C.B.; Ro, S.-H.; Kim, Y.-M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.-H. ULK-Atg13-FIP200 Complexes Mediate mTOR Signaling to the Autophagy Machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Tripathi, D.N.; Chowdhury, R.; Trudel, L.J.; Tee, A.R.; Slack, R.S.; Walker, C.L.; Wogan, G.N. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2–mediated suppression of mTORC1. Proc. Natl. Acad. Sci. USA 2013, 110, E2950–E2957. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Bento, C.F.; Renna, M.; Ghislat, G.; Puri, C.; Ashkenazi, A.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem. 2016, 85, 685–713. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The Role of Atg Proteins in Autophagosome Formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Kim, K.-A.; Shin, D.; Kim, J.-H.; Shin, Y.-J.; Rajanikant, G.; Majid, A.; Baek, S.-H.; Bae, O.-N. Role of Autophagy in Endothelial Damage and Blood–Brain Barrier Disruption in Ischemic Stroke. Stroke 2018, 49, 1571–1579. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Wang, S.; Kroemer, G.; Penninger, J.M.; Uversky, V.N.; Pratico, D.; Henninger, N.; Reiter, R.J.; Bruno, A.; Joshipura, K.; et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 2021, 225, 107848. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, L.; Ju, Y.; Dai, Y. Recent Advances in Metal-Phenolic Networks for Cancer Theranostics. Small 2021, 17, 2100314. [Google Scholar] [CrossRef]
- Shamsi, A.; Anwar, S.; Mohammad, T.; Shahwan, M.; Hassan, I.; Islam, A. Therapeutic Potential of Polyphenols in Alzheimer’s Therapy: Broad-Spectrum and Minimal Side Effects as Key Aspects; Springer: Singapore, 2021; pp. 111–133. [Google Scholar] [CrossRef]
- Xu, H.; Wang, E.; Chen, F.; Xiao, J.; Wang, M. Neuroprotective Phytochemicals in Experimental Ischemic Stroke: Mechanisms and Potential Clinical Applications. Oxid. Med. Cell. Longev. 2021, 2021, 6687386. [Google Scholar] [CrossRef]
- Hamsalakshmi; Alex, A.M.; Marappa, M.A.; Joghee, S.; Chidambaram, S.B. Therapeutic benefits of flavonoids against neuroinflammation: A systematic review. Inflammopharmacology 2022, 30, 111–136. [CrossRef]
- Ulya, T.; Ardianto, C.; Anggreini, P.; Budiatin, A.S.; Setyawan, D.; Khotib, J. Quercetin promotes behavioral recovery and biomolecular changes of melanocortin-4 receptor in mice with ischemic stroke. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 349–355. [Google Scholar] [CrossRef]
- Lee, Y.L.; Park, S.M.; Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.-H.; Lee, C.-H.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shen, Y.-J.; Chen, M.; Zhao, J.-Y.; Chen, S.-H.; Zhang, W.; Song, J.-K.; Li, L.; Du, G.-H. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-J.; Kang, J.-B.; Shah, F.-A.; Jin, Y.-B.; Koh, P.-O. Quercetin Attenuates Decrease of Thioredoxin Expression Following Focal Cerebral Ischemia and Glutamate-induced Neuronal Cell Damage. Neuroscience 2020, 428, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-J.; Jeon, S.-J.; Kang, J.-B.; Koh, P.-O. Quercetin Reduces Ischemic Brain Injury by Preventing Ischemia-induced Decreases in the Neuronal Calcium Sensor Protein Hippocalcin. Neuroscience 2020, 430, 47–62. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chang, C.-Y.; Lin, S.-Y.; Wang, J.-D.; Wu, C.-C.; Chen, W.-Y.; Kuan, Y.-H.; Liao, S.-L.; Wang, W.-Y.; Chen, C.-J. Quercetin protects against cerebral ischemia/reperfusion and oxygen glucose deprivation/reoxygenation neurotoxicity. J. Nutr. Biochem. 2020, 83, 108436. [Google Scholar] [CrossRef]
- Park, D.-J.; Kang, J.-B.; Shah, F.-A.; Koh, P.-O. Quercetin attenuates the reduction of parvalbumin in middle cerebral artery occlusion animal model. Lab. Anim. Res. 2021, 37, 9. [Google Scholar] [CrossRef]
- Park, D.-J.; Shah, F.-A.; Koh, P.-O. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med. Sci. 2018, 80, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Park, D.-J.; Kang, J.-B.; Shah, M.-A.; Koh, P.-O. Quercetin alleviates the injury-induced decrease of protein phosphatase 2A subunit B in cerebral ischemic animal model and glutamate-exposed HT22 cells. J. Vet. Med. Sci. 2019, 81, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Le, K.; Song, Z.; Deng, J.; Peng, X.; Zhang, J.; Wang, L.; Zhou, L.; Bi, H.; Liao, Z.; Feng, Z. Quercetin alleviates neonatal hypoxic-ischemic brain injury by inhibiting microglia-derived oxidative stress and TLR4-mediated inflammation. Inflamm. Res. 2020, 69, 1201–1213. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Kim, M.-O.; Ali-Shah, F.; Koh, P.-O. Quercetin attenuates the injury-induced reduction of γ-enolase expression in a middle cerebral artery occlusion animal model. LAR 2017, 33, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-P.; Shi, Y.-W.; Tang, M.; Zhang, X.-C.; Gu, Y.; Liang, X.-M.; Wang, Z.-W.; Ding, F. Isoquercetin Ameliorates Cerebral Impairment in Focal Ischemia through Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Effects in Primary Culture of Rat Hippocampal Neurons and Hippocampal CA1 Region of Rats. Mol. Neurobiol. 2017, 54, 2126–2142. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem.-Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, L.; Zhang, Y.; Liu, X.; Li, J. Rutin attenuates cerebral ischemia–reperfusion injury in ovariectomized rats via estrogen-receptor-mediated BDNF–TrkB and NGF–TrkA signaling. Biochem. Cell Biol. 2018, 96, 672–681. [Google Scholar] [CrossRef]
- Li, W.-H.; Cheng, X.; Yang, Y.-L.; Liu, M.; Zhang, S.-S.; Wang, Y.-H.; Du, G.-H. Kaempferol attenuates neuroinflammation and blood brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res. 2019, 1722, 146361. [Google Scholar] [CrossRef]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects against Cerebral Ischemia Reperfusion Injury through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-p.; Li, G.-c. Kaempferol Protects Cell Damage in In Vitro Ischemia Reperfusion Model in Rat Neuronal PC12 Cells. BioMed Res. Int. 2020, 2020, 2461079. [Google Scholar] [CrossRef]
- Wu, B.; Luo, H.; Zhou, X.; Cheng, C.-Y.; Lin, L.; Liu, B.-L.; Liu, K.; Li, P.; Yang, H. Succinate-induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 2307–2318. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Liu, D.; Ye, Y.; Xu, L.; Yuan, W.; Zhang, Q. Icariin and mesenchymal stem cells synergistically promote angiogenesis and neurogenesis after cerebral ischemia via PI3K and ERK1/2 pathways. Biomed. Pharmacother. 2018, 108, 663–669. [Google Scholar] [CrossRef]
- Mo, Z.-T.; Zheng, J.; Liao, Y.-L. Icariin inhibits the expression of IL-1β, IL-6 and TNF-α induced by OGD/R through the IRE1/XBP1s pathway in microglia. Pharm. Biol. 2021, 59, 1471–1477. [Google Scholar] [CrossRef]
- Dai, M.; Chen, B.; Wang, X.; Gao, C.; Yu, H. Icariin enhance mild hypothermia-induced neuroprotection via inhibiting the activation of NF-κB in experimental ischemic stroke. Metab. Brain Dis. 2021, 36, 1779–1790. [Google Scholar] [CrossRef]
- Sun, L.; Xu, P.; Fu, T.; Huang, X.; Song, J.; Chen, M.; Tian, X.; Yin, H.; Han, J. Myricetin against ischemic cerebral injury in rat middle cerebral artery occlusion model. Mol. Med. Rep. 2018, 17, 3274–3280. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Hu, X.; Guo, S.; Shi, L.; He, Q.; Zhang, P.; Yu, S.; Zhao, R. Myricetin ameliorated ischemia/reperfusion-induced brain endothelial permeability by improvement of eNOS uncoupling and activation eNOS/NO. J. Pharmacol. Sci. 2019, 140, 62–72. [Google Scholar] [CrossRef]
- Tao, J.; Cui, Y.; Duan, Y.; Zhang, N.; Wang, C.; Zhang, F. Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget 2017, 8, 106283–106295. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.-G.; Feng, Z.-T.; Wang, J.-F.; Fu, Y.; Yang, S.-B.; Zhang, S.-Z.; Huang, W.-F.; Xiong, L.; Zhou, H.-J.; Tao, W. Puerarin protects rat brain against ischemia/reperfusion injury by suppressing autophagy via the AMPK-mTOR-ULK1 signaling pathway. Neural Regen. Res. 2018, 13, 989–998. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wei, H.; Gu, T.; Wang, J.; Wu, Z.; Yang, Q. Genistein Attenuates Acute Cerebral Ischemic Damage by Inhibiting the NLRP3 Inflammasome in Reproductively Senescent Mice. Front. Aging Neurosci. 2020, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.-Y.; Xia, X.; Che, L.; Song, Y.-T. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of Nrf2 expression in ovariectomized rats. Neurol. Res. 2018, 40, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Y.; Liu, Y.; Gong, Y.-F.; Zheng, X.-Y. A preliminary report: Genistein attenuates cerebral ischemia injury in ovariectomized rats via regulation of the PI3K-Akt-mTOR pathway. Gen. Physiol. Biophys. 2019, 38, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhou, M.; Chen, M.; Lu, Y.; Shi, D.; Wang, J.; Liu, C. Neuroprotective Effect of Daidzein Extracted from Pueraria lobate Radix in a Stroke Model Via the Akt/mTOR/BDNF Channel. Front. Pharmacol. 2022, 12, 772485. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Yang, Y.; Wang, R.; Wang, Y.; Wu, C.; Du, G. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. 2019, 117, 109102. [Google Scholar] [CrossRef]
- Yuan, Y.; Men, W.; Shan, X.; Zhai, H.; Qiao, X.; Geng, L.; Li, C. Baicalein exerts neuroprotective effect against ischaemic/reperfusion injury via alteration of NF-kB and LOX and AMPK/Nrf2 pathway. Inflammopharmacology 2020, 28, 1327–1341. [Google Scholar] [CrossRef]
- Ran, Y.; Qie, S.; Gao, F.; Ding, Z.; Yang, S.; Tian, G.; Liu, Z.; Xi, J. Baicalein ameliorates ischemic brain damage through suppressing proinflammatory microglia polarization via inhibiting the TLR4/NF-κB and STAT1 pathway. Brain Res. 2021, 1770, 147626. [Google Scholar] [CrossRef]
- Li, W.-H.; Yang, Y.-L.; Cheng, X.; Liu, M.; Zhang, S.-S.; Wang, Y.-H.; Du, G.-H. Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. Apoptosis 2020, 25, 354–369. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Fei, L.; Zhang, Y.; Pang, J.; Gao, W.; Fan, F.; Xing, Y.; Li, X. Baicalein-ameliorated cerebral ischemia-reperfusion injury dependent on calpain 1/AIF pathway. Biosci. Biotechnol. Biochem. 2022, 86, 305–312. [Google Scholar] [CrossRef]
- Song, X.; Gong, Z.; Liu, K.; Kou, J.; Liu, B.; Liu, K. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox Biol. 2020, 34, 101559. [Google Scholar] [CrossRef]
- Chen, H.-L.; Jia, W.-J.; Li, H.-E.; Han, H.; Li, F.; Zhang, X.-L.; Li, J.-J.; Yuan, Y.; Wu, C.-Y. Scutellarin Exerts Anti-Inflammatory Effects in Activated Microglia/Brain Macrophage in Cerebral Ischemia and in Activated BV-2 Microglia through Regulation of MAPKs Signaling Pathway. Neuromol. Med. 2020, 22, 264–277. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Li, D. Breviscapine Alleviates Cognitive Impairments Induced by Transient Cerebral Ischemia/Reperfusion through Its Anti-Inflammatory and Anti-Oxidant Properties in a Rat Model. ACS Chem. Neurosci. 2020, 11, 4489–4498. [Google Scholar] [CrossRef]
- Sun, J.-B.; Li, Y.; Cai, Y.-F.; Huang, Y.; Liu, S.; Yeung, P.K.; Deng, M.-Z.; Sun, G.-S.; Zilundu, P.L.; Hu, Q.-S.; et al. Scutellarin protects oxygen/glucose-deprived astrocytes and reduces focal cerebral ischemic injury. Neural Regen. Res. 2018, 13, 1396–1407. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, T.; He, H.; Yang, L.; Deng, Y. Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed. Pharmacother. 2017, 90, 69–76. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Z.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Li, J.; Xie, X.; Pang, X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 66, 309–316. [Google Scholar] [CrossRef]
- Liu, S.; Su, Y.; Sun, B.; Hao, R.; Pan, S.; Gao, X.; Dong, X.; Ismail, A.M.; Han, B. Luteolin Protects Against CIRI, Potentially via Regulation of the SIRT3/AMPK/mTOR Signaling Pathway. Neurochem. Res. 2020, 45, 2499–2515. [Google Scholar] [CrossRef]
- Sarkaki, A.; Farbood, Y.; Mansouri, M.T.; Badavi, M.; Khorsandi, L.; Dehcheshmeh, M.G.; Shooshtari, M.K. Chrysin prevents cognitive and hippocampal long-term potentiation deficits and inflammation in rat with cerebral hypoperfusion and reperfusion injury. Life Sci. 2019, 226, 202–209. [Google Scholar] [CrossRef]
- Li, T.-F.; Ma, J.; Han, X.-W.; Jia, Y.-X.; Yuan, H.-F.; Shui, S.-F.; Guo, D.; Yan, L. Chrysin ameliorates cerebral ischemia/reperfusion (I/R) injury in rats by regulating the PI3K/Akt/mTOR pathway. Neurochem. Int. 2019, 129, 104496. [Google Scholar] [CrossRef]
- El Khashab, I.H.; Abdelsalam, R.M.; Elbrairy, A.I.; Attia, A.S. Chrysin attenuates global cerebral ischemic reperfusion injury via suppression of oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2019, 112, 108619. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, Y.; Chen, X.; Zhao, K.; Zhai, Q.; Tu, F. Apigenin Protects the Brain against Ischemia/Reperfusion Injury via Caveolin-1/VEGF In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2018, 2018, 7017204. [Google Scholar] [CrossRef]
- Ling, C.; Lei, C.; Zou, M.; Cai, X.; Xiang, Y.; Xie, Y.; Li, X.; Huang, D.; Wang, Y. Neuroprotective effect of apigenin against cerebral ischemia/reperfusion injury. J. Int. Med. Res. 2020, 48, 0300060520945859. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Chen, K.; Liu, T.; Guo, W.; Xu, Z. Epigallocatechin-3-Gallate Reduces Neuronal Apoptosis in Rats after Middle Cerebral Artery Occlusion Injury via PI3K/AKT/eNOS Signaling Pathway. BioMed Res. Int. 2018, 2018, 6473580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-C.; Xu, H.; Yuan, Y.; Chen, J.-Y.; Zhang, Y.-J.; Lin, Y.; Yuan, S.-Y. Delayed Treatment with Green Tea Polyphenol EGCG Promotes Neurogenesis after Ischemic Stroke in Adult Mice. Mol. Neurobiol. 2017, 54, 3652–3664. [Google Scholar] [CrossRef]
- Park, D.-J.; Kang, J.-B.; Koh, P.-O. Epigallocatechin gallate alleviates neuronal cell damage against focal cerebral ischemia in rats. J. Vet. Med. Sci. 2020, 82, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Park, D.-J.; Kang, J.-B.; Shah, M.-A.; Koh, P.-O. Epigallocatechin Gallate Alleviates Down-Regulation of Thioredoxin in Ischemic Brain Damage and Glutamate-Exposed Neuron. Neurochem. Res. 2021, 46, 3035–3049. [Google Scholar] [CrossRef]
- Fu, B.; Zeng, Q.; Zhang, Z.; Qian, M.; Chen, J.; Dong, W.; Li, M. Epicatechin Gallate Protects HBMVECs from Ischemia/Reperfusion Injury through Ameliorating Apoptosis and Autophagy and Promoting Neovascularization. Oxid. Med. Cell. Longev. 2019, 2019, 7824684. [Google Scholar] [CrossRef]
- Yang, B.; Sun, Y.; Lv, C.; Zhang, W.; Chen, Y. Procyanidins exhibits neuroprotective activities against cerebral ischemia reperfusion injury by inhibiting TLR4-NLRP3 inflammasome signal pathway. Psychopharmacology 2020, 237, 3283–3293. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, J.; Huang, L.; Luo, N.; Liang, X.; Liang, M.; Xie, W. Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects. Clin. Exp. Pharmacol. Physiol. 2017, 44, 862–871. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Lu, S.; Li, W.; Yang, D.; Su, W.; Wang, X.; Shen, J. Naringin Attenuates Cerebral Ischemia-Reperfusion Injury through Inhibiting Peroxynitrite-Mediated Mitophagy Activation. Mol. Neurobiol. 2018, 55, 9029–9042. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Wu, F.; Chi, X.; Pang, Y.; Jin, H.; Sun, Y.; Zhang, S. Neuroprotective Effects of Hesperetin in Regulating Microglia Polarization after Ischemic Stroke by Inhibiting TLR4/NF-κB Pathway. J. Healthc. Eng. 2021, 2021, 9938874. [Google Scholar] [CrossRef]
- Xu, B.; He, X.; Sui, Y.; Wang, X.; Wang, X.; Ren, L.; Zhai, Y.-X. Ginkgetin aglycone attenuates neuroinflammation and neuronal injury in the rats with ischemic stroke by modulating STAT3/JAK2/SIRT1. Folia Neuropathol. 2019, 57, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-X.; Chen, J.-H.; Li, J.-W.; Cheng, F.-R.; Yuan, K. Protection of Anthocyanin from Myrica rubra against Cerebral Ischemia-Reperfusion Injury via Modulation of the TLR4/NF-κB and NLRP3 Pathways. Molecules 2018, 23, 17880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement. Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Cui, M.; Dai, G.; Yuan, T.; Li, Y.; Ji, T.; Pan, Y. Protective Effect of Anthocyanin on Neurovascular Unit in Cerebral Ischemia/Reperfusion Injury in Rats. Front. Neurosci. 2018, 12, 947. [Google Scholar] [CrossRef]
- Cai, Y.; Li, X.; Pan, Z.; Zhu, Y.; Tuo, J.; Meng, Q.; Dai, G.; Yang, G.; Pan, Y. Anthocyanin ameliorates hypoxia and ischemia induced inflammation and apoptosis by increasing autophagic flux in SH-SY5Y cells. Eur. J. Pharmacol. 2020, 883, 173360. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, A.; Lang, D.K.; Bhatia, S.; Al-Harrasi, A.; Aleya, L.; Behl, T. Potential of flavonoids as anti-Alzheimer’s agents: Bench to bedside. Environ. Sci. Pollut. Res. 2022, 29, 26063–26077. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, G.; Kumar, R.; Singh, B.; Malik, R.; Katare, O.P.; Raza, K. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: Biochemical, pharmacokinetic and biodistribution evidences. Int. J. Pharm. 2016, 515, 307–314. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-β-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef]
- Scott, E.; Zhang, Q.-G.; Wang, R.; Vadlamudi, R.; Brann, D. Estrogen neuroprotection and the critical period hypothesis. Front. Neuroendocrinol. 2012, 33, 85–104. [Google Scholar] [CrossRef] [Green Version]
- Renoux, C.; Suissa, S. Hormone Therapy Administration in Postmenopausal Women and Risk of Stroke. Women’s Health 2011, 7, 355–361. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms. Biomed. Pharmacother. 2022, 147, 112642. [Google Scholar] [CrossRef]
- Yuan, J.-Y.; Tong, Z.-Y.; Dong, Y.-C.; Zhao, J.-Y.; Shang, Y. Research progress on icariin, a traditional Chinese medicine extract, in the treatment of asthma. Allergol. Immunopathol. 2022, 50, 9–16. [Google Scholar] [CrossRef]
- Chuang, Y.; Van, I.; Zhao, Y.; Xu, Y. Icariin ameliorate Alzheimer’s disease by influencing SIRT1 and inhibiting Aβ cascade pathogenesis. J. Chem. Neuroanat. 2021, 117, 102014. [Google Scholar] [CrossRef]
- Wei, S.-Y.; Chen, Y.; Xu, X.-Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Leonard, L.M.; Choi, M.S.; Cross, T.-W.L. Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients 2022, 14, 553. [Google Scholar] [CrossRef]
- Lee, A.W.; Poynor, V.; McEligot, A.J. Urinary Phytoestrogen Levels Are Associated with Female Hormonal Cancers: An Analysis of NHANES Data from 1999 to 2010. Nutr. Cancer 2022, 1–9. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Liang, W.; Huang, X.; Chen, W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Rangarajan, P.; Kan, E.M.; Wu, Y.; Wu, C.; Ling, E.-A. Scutellarin regulates the Notch pathway and affects the migration and morphological transformation of activated microglia in experimentally induced cerebral ischemia in rats and in activated BV-2 microglia. J. Neuroinflamm. 2015, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Bordet, R.; Ouk, T.; Petrault, O.; Gelé, P.; Gautier, S.; Laprais, M.; Deplanque, D.; Duriez, P.; Staels, B.; Fruchart, J.C.; et al. PPAR: A new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem. Soc. Trans. 2006, 34, 1341–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.-J.; Gao, Y.; Bennett, M.V.; et al. Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018, 163–164, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef]
- Yu, S.-W.; Andrabi, S.A.; Wang, H.; Kim, N.S.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 18314–18319. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 176, 113870. [Google Scholar] [CrossRef]
- Chen, J.-L.; Duan, W.-J.; Luo, S.; Li, S.; Ma, X.-H.; Hou, B.-N.; Cheng, S.-Y.; Fang, S.-H.; Wang, Q.; Huang, S.-Q.; et al. Ferulic acid attenuates brain microvascular endothelial cells damage caused by oxygen-glucose deprivation via punctate-mitochondria-dependent mitophagy. Brain Res. 2017, 1666, 17–26. [Google Scholar] [CrossRef]
- Cheng, C.; Kao, S.; Lee, Y. Ferulic acid ameliorates cerebral infarction by activating Akt/mTOR/4E-BP1/Bcl-2 anti-apoptotic signaling in the penumbral cortex following permanent cerebral ischemia in rats. Mol. Med. Rep. 2019, 19, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-Y.; Kao, S.-T.; Lee, Y.-C. Ferulic Acid Exerts Anti-apoptotic Effects against Ischemic Injury by Activating HSP70/Bcl-2- and HSP70/Autophagy-Mediated Signaling after Permanent Focal Cerebral Ischemia in Rats. Am. J. Chin. Med. 2019, 47, 39–61. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Cui, H.-Y.; Yang, Y.; Zhang, C.; Zhu, C.-H.; Miao, J.-Y.; Chen, R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018, 13, 2119–2128. [Google Scholar] [CrossRef]
- Miao, M.; Cao, L.; Li, R.; Fang, X.; Miao, Y. Protective effect of chlorogenic acid on the focal cerebral ischemia reperfusion rat models. Saudi Pharm. J. 2017, 25, 556–563. [Google Scholar] [CrossRef]
- Shah, M.-A.; Kang, J.-B.; Park, D.-J.; Kim, M.-O.; Koh, P.-O. Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models. Neurosci. Lett. 2021, 760, 136085. [Google Scholar] [CrossRef]
- Roshan-Milani, S.; Sattari, P.; Ghaderi-Pakdel, F.; Naderi, R. miR-23b/TAB3/NF-κB/p53 axis is involved in hippocampus injury induced by cerebral ischemia–reperfusion in rats: The protective effect of chlorogenic acid. BioFactors 2022, in press. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Dev. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.-A.; Kang, J.-B.; Park, D.-J.; Kim, M.-O.; Koh, P.-O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neurosci. Lett. 2022, 773, 136495. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.-K.; Zhang, X.; Zhou, Q.-M.; He, G.-R.; Xu, X.-N.; Rong, Y.; Zhou, W.-X.; Du, G.-H. Salvianolic acid A attenuates ischemia reperfusion induced rat brain damage by protecting the blood brain barrier through MMP-9 inhibition and anti-inflammation. Chin. J. Nat. Med. 2018, 16, 184–193. [Google Scholar] [CrossRef]
- Song, J.; Zhang, W.; Wang, J.; Yang, H.; Zhou, Q.; Wang, H.; Li, L.; Du, G. Inhibition of FOXO3a/BIM signaling pathway contributes to the protective effect of salvianolic acid A against cerebral ischemia/reperfusion injury. Acta Pharm. Sin. B 2019, 9, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Fang, G. Salvianolic acid A attenuates cerebral ischemia/reperfusion injury induced rat brain damage, inflammation and apoptosis by regulating miR-499a/DDK1. Am. J. Transl. Res. 2020, 12, 3288–3301. [Google Scholar] [PubMed]
- Liu, C.-D.; Liu, N.-N.; Zhang, S.; Ma, G.-D.; Yang, H.-G.; Kong, L.-L.; Du, G.-H. Salvianolic acid A prevented cerebrovascular endothelial injury caused by acute ischemic stroke through inhibiting the Src signaling pathway. Acta Pharmacol. Sin. 2021, 42, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Jin, L.; Ma, Q.; Huang, Y.; Yang, Q.; Chen, M.; Shou, Q. Salvianolic acid A alleviated inflammatory response mediated by microglia through inhibiting the activation of TLR2/4 in acute cerebral ischemia-reperfusion. Phytomedicine 2021, 87, 153569. [Google Scholar] [CrossRef]
- Zhang, S.; Kong, D.-W.; Ma, G.-D.; Liu, C.-D.; Yang, Y.-J.; Liu, S.; Jiang, N.; Pan, Z.-R.; Zhang, W.; Kong, L.-L.; et al. Long-term administration of salvianolic acid A promotes endogenous neurogenesis in ischemic stroke rats through activating Wnt3a/GSK3β/β-catenin signaling pathway. Acta Pharmacol. Sin. 2022. [Google Scholar] [CrossRef]
- Xu, S.; Zhong, A.; Ma, H.; Li, D.; Hu, Y.; Xu, Y.; Zhang, J. Neuroprotective effect of salvianolic acid B against cerebral ischemic injury in rats via the CD40/NF-κB pathway associated with suppression of platelets activation and neuroinflammation. Brain Res. 2017, 1661, 37–48. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Gu, T.; Yu, D.; Shi, Y.; Gao, Z.; Wang, Z.; Liu, W.; Fan, Z.; Hou, W.; et al. Astrocytic glycogen mobilization participates in salvianolic acid B-mediated neuroprotection against reperfusion injury after ischemic stroke. Exp. Neurol. 2022, 349, 113966. [Google Scholar] [CrossRef]
- Kale, S.; Sarode, L.P.; Kharat, A.; Ambulkar, S.; Prakash, A.; Sakharkar, A.J.; Ugale, R.R. Protocatechuic Acid Prevents Early Hour Ischemic Reperfusion Brain Damage by Restoring Imbalance of Neuronal Cell Death and Survival Proteins. J. Stroke Cerebrovasc. Dis. 2021, 30, 105507. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, L.; Mao, Y. Gallic acid attenuates cerebral ischemia/re-perfusion-induced blood–brain barrier injury by modifying polarization of microglia. J. Immunotoxicol. 2022, 19, 17–26. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Sarkaki, A.; Rashno, M.; Farbood, Y. Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Chen, A.; Cheng, X.; Zhang, G.; Sun, J.; Zhao, Y.; Huang, Y.; Zhu, Y. Rhein protects against cerebral ischemic-/reperfusion-induced oxidative stress and apoptosis in rats. Int. J. Mol. Med. 2018, 41, 2802–2812. [Google Scholar] [CrossRef]
- Neto-Neves, E.M.; Filho, C.D.S.M.B.; Dejani, N.N.; de Sousa, D.P. Ferulic Acid and Cardiovascular Health: Therapeutic and Preventive Potential. Mini-Rev. Med. Chem. 2021, 21, 1625–1637. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan—Rosmarinic acid conjugates with antioxidant, anti-inflammatory and photoprotective properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Yang, M.; Orgah, J.; Zhu, J.; Fan, G.; Han, J.; Wang, X.; Zhang, B.; Zhu, Y. Danhong injection attenuates cardiac injury induced by ischemic and reperfused neuronal cells through regulating arginine vasopressin expression and secretion. Brain Res. 2016, 1642, 516–523. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Li, X.; Chen, L.; Wang, H.; Wu, J.; Zheng, J.; Wu, D. Characterization of the Kremen-binding Site on Dkk1 and Elucidation of the Role of Kremen in Dkk-mediated Wnt Antagonism*. J. Biol. Chem. 2008, 283, 23371–23375. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Liang, Q.-L.; Luo, G.-A.; Zhao, Z.-Z.; Jiang, Z.-H. Multi-component HPLC Fingerprinting of Radix Salviae Miltiorrhizae and Its LC-MS-MS Identification. Chem. Pharm. Bull. 2005, 53, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.P.; Zhao, X.M.; Pan, S.D.; Guo, D.A.; Wei, R.; Han, J.J.; Kainoh, M.; Xia, Z.L.; de Groot, P.G.; Lisman, T. Salvianolic Acid B inhibits platelet adhesion under conditions of flow by a mechanism involving the collagen receptor α2β1. Thromb. Res. 2008, 123, 298–305. [Google Scholar] [CrossRef]
- Sun, Y.; Kotani, A.; Machida, K.; Yamamoto, K.; Hakamata, H. Determination of Phenolic Compounds in Beverages by Three-Flow Channel Isocratic HPLC with Electrochemical Detections Using a Column-Switching Technique. Chem. Pharm. Bull. 2022, 70, 43–49. [Google Scholar] [CrossRef]
- Loarca-Piña, G.; Mendoza, S.; Ramos-Gómez, M.; Reynoso, R. Antioxidant, Antimutagenic, and Antidiabetic Activities of Edible Leaves from Cnidoscolus chayamansa Mc. Vaugh. J. Food Sci. 2010, 75, H68–H72. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Men, L.; Sun, Y.; Wei, M.; Fan, X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. Eur. J. Pharmacol. 2021, 892, 173796. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Zhu, Y.; Wang, K.; Wang, Y. Effect of magnolol on cerebral injury and blood brain barrier dysfunction induced by ischemia-reperfusion in vivo and in vitro. Metab. Brain Dis. 2017, 32, 1109–1118. [Google Scholar] [CrossRef]
- Kou, D.-Q.; Jiang, Y.-L.; Qin, J.-H.; Huang, Y.-H. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol. Rep. 2017, 69, 642–647. [Google Scholar] [CrossRef]
- Huang, S.; Tai, S.; Chang, C.; Tu, Y.; Chang, C.; Lee, E. Magnolol protects against ischemic-reperfusion brain damage following oxygen-glucose deprivation and transient focal cerebral ischemia. Int. J. Mol. Med. 2018, 41, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xie, J.; Lin, K.; Qi, L. Influencing mechanism of magnolol on expression of BDNF and Bax in rats with cerebral ischemic stroke. Exp. Ther. Med. 2018, 16, 4423–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, W.; Gouda, M.; Cai, E.; Wang, R.; Xu, W.; Wu, Y.; Munekata, P.E.S.; Lorenzo, J.M. The Antioxidant Phytochemical Schisandrin A Promotes Neural Cell Proliferation and Differentiation after Ischemic Brain Injury. Molecules 2021, 26, 7466. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Elkin, K.; Shi, Y.; Zhang, Z.; Cheng, Y.; Gu, J.; Liang, J.; Wang, C.; Ji, X. Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-κB signaling pathway inhibition. Neurol. Res. 2020, 42, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-J.; Xie, G.-N.; Liu, L.; Fu, Z.-J.; Zhang, Z.-W.; Teng, L.-Z. Sesamol attenuates oxidative stress, apoptosis and inflammation in focal cerebral ischemia/reperfusion injury. Exp. Ther. Med. 2017, 14, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Jiang, L.; Che, F.; Lu, Y.; Xie, Z.; Wang, H. Arctigenin attenuates ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2017, 493, 821–826. [Google Scholar] [CrossRef]
- Thundyil, J.; Manzanero, S.; Pavlovski, D.; Cully, T.R.; Lok, K.-Z.; Widiapradja, A.; Chunduri, P.; Jo, D.-G.; Naruse, C.; Asano, M.; et al. Evidence That the EphA2 Receptor Exacerbates Ischemic Brain Injury. PLoS ONE 2013, 8, e53528. [Google Scholar] [CrossRef] [Green Version]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W. Resveratrol Alleviates Ischemic Brain Injury by Inhibiting the Activation of Pro-Inflammatory Microglia Via the CD147/MMP-9 Pathway. J. Stroke Cerebrovasc. Dis. 2022, 31, 106307. [Google Scholar] [CrossRef]
- Chang, C.; Zhao, Y.; Song, G.; She, K. Resveratrol protects hippocampal neurons against cerebral ischemia-reperfusion injury via modulating JAK/ERK/STAT signaling pathway in rats. J. Neuroimmunol. 2018, 315, 9–14. [Google Scholar] [CrossRef]
- Lei, J.; Chen, Q. Resveratrol attenuates brain damage in permanent focal cerebral ischemia via activation of PI3K/Akt signaling pathway in rats. Neurol. Res. 2018, 40, 1014–1020. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef]
- Dou, Z.; Rong, X.; Zhao, E.; Zhang, L.; Lv, Y. Neuroprotection of Resveratrol against Focal Cerebral Ischemia/Reperfusion Injury in Mice through a Mechanism Targeting Gut-Brain Axis. Cell. Mol. Neurobiol. 2019, 39, 883–898. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, N.; Singh, T.G. Effects of resveratrol postconditioning on cerebral ischemia in mice: Role of the sirtuin-1 pathway. Can. J. Physiol. Pharmacol. 2019, 97, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Pineda-Ramírez, N.; Alquisiras-Burgos, I.; Ortiz-Plata, A.; Ruiz-Tachiquín, M.-E.; Espinoza-Rojo, M.; Aguilera, P. Resveratrol Activates Neuronal Autophagy through AMPK in the Ischemic Brain. Mol. Neurobiol. 2020, 57, 1055–1069. [Google Scholar] [CrossRef]
- Teertam, S.K.; Jha, S.; Babu, P.P. Up-regulation of Sirt1/miR-149-5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J. Clin. Neurosci. 2020, 72, 402–411. [Google Scholar] [CrossRef]
- Ma, S.; Fan, L.; Li, J.; Zhang, B.; Yan, Z. Resveratrol promoted the M2 polarization of microglia and reduced neuroinflammation after cerebral ischemia by inhibiting miR-155. Int. J. Neurosci. 2020, 130, 817–825. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Li, Y.; Li, W. Resveratrol attenuates cerebral ischaemia reperfusion injury via modulating mitochondrial dynamics homeostasis and activating AMPK-Mfn1 pathway. Int. J. Exp. Pathol. 2019, 100, 337–349. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Tang, F.; Guo, S.; Liao, H.; Fan, C.; Yang, Q. Resveratrol-mediated neurorestoration after cerebral ischemic injury—Sonic Hedgehog signaling pathway. Life Sci. 2021, 280, 119715. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ren, D.; Feng, X.; Huang, J.; Wang, D.; Li, T.; Zhang, D. Neuroprotective and Anti-Inflammatory Effect of Pterostilbene Against Cerebral Ischemia/Reperfusion Injury via Suppression of COX-2. Front. Pharmacol. 2021, 12, 770329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Mi, Y.; Yang, Y.; Li, Q.; Zhou, D.; Wei, K.; Chen, G.; Li, N.; Hou, Y. Pterostilbene alleviates cerebral ischemia and reperfusion injury in rats by modulating microglial activation. Food Funct. 2020, 11, 5432–5445. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, X.; Luo, J.; Wang, X.; Guo, H.; Feng, D.; Zhao, L.; Bai, H.; Song, M.; Liu, X.; et al. Pterostilbene Attenuates Astrocytic Inflammation and Neuronal Oxidative Injury after Ischemia-Reperfusion by Inhibiting NF-κB Phosphorylation. Front. Immunol. 2019, 10, 2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, W.; Liu, J.; Cui, Y.; Cui, J. Piceatannol protects against cerebral ischemia/reperfusion-induced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep. 2020, 22, 5399–5411. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.; Ye, J.; Pan, Z.; Hu, P.; Zhong, X.; Qiu, F.; Zhang, D.; Huang, Z. Protective Effect of Piceatannol against Cerebral Ischaemia–Reperfusion Injury Via Regulating Nrf2/HO-1 Pathway In Vivo and Vitro. Neurochem. Res. 2021, 46, 1869–1880. [Google Scholar] [CrossRef]
- Ruan, W.; Li, J.; Xu, Y.; Wang, Y.; Zhao, F.; Yang, X.; Jiang, H.; Zhang, L.; Saavedra, J.M.; Shi, L.; et al. MALAT1 Up-Regulator Polydatin Protects Brain Microvascular Integrity and Ameliorates Stroke through C/EBPβ/MALAT1/CREB/PGC-1α/PPARγ Pathway. Cell. Mol. Neurobiol. 2019, 39, 265–286. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Z.; Zhang, S.; Nanda, A.; Li, G. CD147: A novel modulator of inflammatory and immune disorders. Curr. Med. Chem. 2014, 21, 2138–2145. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, W.; Lin, Q.; Wang, W. Icariin influences cardiac remodeling following myocardial infarction by regulating the CD147/MMP-9 pathway. J. Int. Med. Res. 2018, 46, 2371–2385. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Xiao, A.Y.; Chen, R.; Granger, D.N.; Li, G. Inhibition of CD147 (Cluster of Differentiation 147) Ameliorates Acute Ischemic Stroke in Mice by Reducing Thromboinflammation. Stroke 2017, 48, 3356–3365. [Google Scholar] [CrossRef]
- Khoury, N.; Xu, J.; Stegelmann, S.D.; Jackson, C.W.; Koronowski, K.B.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Induces Genomic and Metabolic Adaptations within the Long-Term Window of Cerebral Ischemic Tolerance Leading to Bioenergetic Efficiency. Mol. Neurobiol. 2019, 56, 4549–4565. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Khoury, N.; Saul, I.; Loris, Z.B.; Cohan, C.H.; Stradecki-Cohan, H.M.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke 2017, 48, 3117–3125. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Qu, Y.; Ding, Y. Curcumin Alleviates Oxygen-Glucose-Deprivation/Reperfusion-Induced Oxidative Damage by Regulating miR-1287-5p/LONP2 Axis in SH-SY5Y Cells. Anal. Cell. Pathol. 2021, 2021, 5548706. [Google Scholar] [CrossRef]
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-κB Suppression and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2021, 2021, 1552127. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.-H.; Zhou, L.; Ding, X.-L.; Meng, Y.-C.; Han, K. Curcumin alleviates OGD/R-induced PC12 cell damage via repressing CCL3 and inactivating TLR4/MyD88/MAPK/NF-κB to suppress inflammation and apoptosis. J. Pharm. Pharmacol. 2020, 72, 1176–1185. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J. Curcumin Attenuates Cerebral Ischemia-reperfusion Injury Through Regulating Mitophagy and Preserving Mitochondrial Function. Curr. Neurovasc. Res. 2020, 17, 113–122. [Google Scholar] [CrossRef]
- Mo, Y.; Yue, E.; Shi, N.; Liu, K. The protective effects of curcumin in cerebral ischemia and reperfusion injury through PKC-θ signaling. Cell Cycle 2021, 20, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, T.; Qi, W.; Li, Y.; Gu, J.; Liu, C.; Sha, Y.; Yang, B.; Hu, S.; Zong, X. Curcumin ameliorates ischemic stroke injury in rats by protecting the integrity of the blood-brain barrier. Exp. Ther. Med. 2021, 22, 783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ran, Y.; Huang, S.; Wen, S.; Zhang, W.; Liu, X.; Ji, Z.; Geng, X.; Ji, X.; Du, H.; et al. Curcumin Protects against Ischemic Stroke by Titrating Microglia/Macrophage Polarization. Front. Aging Neurosci. 2017, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Mol. Med. Rep. 2017, 16, 4710–4720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Fan, Y.; Sun, H.; Chen, L.; Man, X. Curcumin inhibits endoplasmic reticulum stress induced by cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2017, 14, 4047–4052. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin against Cerebral Ischemia-Reperfusion Via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Xu, L.; Ding, L.; Su, Y.; Shao, R.; Liu, J.; Huang, Y. Neuroprotective effects of curcumin against rats with focal cerebral ischemia-reperfusion injury. Int. J. Mol. Med. 2019, 43, 1879–1887. [Google Scholar] [CrossRef]
- Xu, H.; Nie, B.; Liu, L.; Zhang, C.; Zhang, Z.; Xu, M.; Mei, Y. Curcumin Prevents Brain Damage and Cognitive Dysfunction During Ischemic-reperfusion through the Regulation of miR-7-5p. Curr. Neurovasc. Res. 2019, 16, 441–454. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Feng, J. The neuroprotective effects of curcumin are associated with the regulation of the reciprocal function between autophagy and HIF-1 alpha in cerebral ischemia-reperfusion injury. Drug Des. Dev. Ther. 2019, 13, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.-J.; Gu, A.-P.; Cai, J.; Wu, Y.; Chen, R.-C. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018, 8, e00921. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Yang, L.; Yuan, J.; Jia, J.; Yang, S. SOD2 Mediates Curcumin-Induced Protection against Oxygen-Glucose Deprivation/Reoxygenation Injury in HT22 Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 2160642. [Google Scholar] [CrossRef]
- Xia, M.; Ye, Z.; Shi, Y.; Zhou, L.; Hua, Y. Curcumin improves diabetes mellitus-associated cerebral infarction by increasing the expression of GLUT1 and GLUT3. Mol. Med. Rep. 2018, 17, 1963–1969. [Google Scholar] [CrossRef] [Green Version]
- Subedi, L.; Gaire, B.P. Neuroprotective Effects of Curcumin in Cerebral Ischemia: Cellular and Molecular Mechanisms. ACS Chem. Neurosci. 2021, 12, 2562–2572. [Google Scholar] [CrossRef]
- Henke, N.; Albrecht, P.; Bouchachia, I.; Ryazantseva, M.; Knoll, K.; Lewerenz, J.; Kaznacheyeva, E.; Maher, P.; Methner, A. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 2013, 4, e470. [Google Scholar] [CrossRef] [Green Version]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Nutraceutical nanodelivery; an insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit. Rev. Food Sci. Nutr. 2021, 61, 3031–3065. [Google Scholar] [CrossRef]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- Mrvová, N.; Škandík, M.; Kuniaková, M.; Račková, L. Modulation of BV-2 microglia functions by novel quercetin pivaloyl ester. Neurochem. Int. 2015, 90, 246–254. [Google Scholar] [CrossRef]
- Škandík, M.; Mrvová, N.; Bezek, Š.; Račková, L. Semisynthetic quercetin-quinone mitigates BV-2 microglia activation through modulation of Nrf2 pathway. Free Radic. Biol. Med. 2020, 152, 18–32. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, T.; Zheng, B.; Zhang, Y.; Li, X.; Zhang, F.; Cen, J.; Duan, S. Curcumin Derivative Cur20 Attenuated Cerebral Ischemic Injury by Antioxidant Effect and HIF-1α/VEGF/TFEB-Activated Angiogenesis. Front. Pharmacol. 2021, 12, 648107. [Google Scholar] [CrossRef]
- Yan, G.; Wang, Y.; Han, X.; Zhang, Q.; Xie, H.; Chen, J.; Ji, D.; Mao, C.; Lu, T. A Modern Technology Applied in Traditional Chinese Medicine: Progress and Future of the Nanotechnology in TCM. Dose-Response 2019, 17, 1559325819872854. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sarkar, S.; Mandal, A.K.; Das, N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS ONE 2013, 8, e57735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Sarkar, S.; Choudhury, S.T.; Ghosh, T.; Das, N. Triphenyl phosphonium coated nano-quercetin for oral delivery: Neuroprotective effects in attenuating age related global moderate cerebral ischemia reperfusion injury in rats. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood–brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef]
- Kinfe, T.; Stadlbauer, A.; Winder, K.; Hurlemann, R.; Buchfelder, M. Incisionless MR-guided focused ultrasound: Technical considerations and current therapeutic approaches in psychiatric disorders. Expert Rev. Neurother. 2020, 20, 687–696. [Google Scholar] [CrossRef]
- McDannold, N.; Zhang, Y.; Supko, J.G.; Power, C.; Sun, T.; Peng, C.; Vykhodtseva, N.; Golby, A.J.; Reardon, D.A. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics 2019, 9, 6284–6299. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liu, Z.; Cai, F.; Niu, W.; Song, L.; Liang, H.; Su, Z.; Yu, B.; Yan, F. Brain Delivery of Curcumin through Low-Intensity Ultrasound-Induced Blood-Brain Barrier Opening via Lipid-PLGA Nanobubbles. Int. J. Nanomed. 2021, 16, 7433–7447. [Google Scholar] [CrossRef]
- Neuhaus, A.; Couch, Y.; Hadley, G.; Buchan, A.M. Neuroprotection in stroke: The importance of collaboration and reproducibility. Brain 2017, 140, 2079–2092. [Google Scholar] [CrossRef] [Green Version]
- Kalra, P.; Khan, H.; Kaur, A.; Singh, T.G. Mechanistic Insight on Autophagy Modulated Molecular Pathways in Cerebral Ischemic Injury: From Preclinical to Clinical Perspective. Neurochem. Res. 2022, 47, 825–843. [Google Scholar] [CrossRef]
- Moretti, A.; Ferrari, F.; Villa, R.F. Neuroprotection for ischaemic stroke: Current status and challenges. Pharmacol. Ther. 2015, 146, 23–34. [Google Scholar] [CrossRef]


| Polyphenolic Compound | Chemical Structure | Models and Treatments | Observed Effects | Mechanisms | Reference | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| Flavonols | ||||||
| Quercetin |  | (Not available) NA | ICR mice Transient-middle cerebral artery occlusion (T-MCAO) intraperitoneal injection (i.p.) for 7 days before T-MCAO 100, 150, 200 mg/kg | Improved behavioral functions Reduced BBB permeability | ↑mRNA MC4R | [106] |
| NA | Gerbils T-MCAO Intragastric injection (i.g.) for 15 days before T-MCAO 20 mg/kg | Anti-oxidative stress | ↑SOD1 ↑SOD2 ↑CAT ↑GPx | [107] | ||
| NA | Wistar rats T-MCAO i.p. immediately after reperfusion 10, 30, 50 mg/kg | Anti-oxidative stress Reduced BBB permeability | ↑Sirt1/Nrf2/HO-1 | [108] | ||
| HT22 cell line and primary cortical neurons Glutamate stimulated 1, 3, 5 µM | Sprague Dawley (SD) rats permanent-MCAO (P-MCAO) i.p. 0.5 h before P-MCAO 10 mg/kg | Suppressed glutamate-induced oxidative stress | ↑Thioredoxin/ASK-1 ↓Caspase-3 | [109] | ||
| HT22 cell line Glutamate stimulated (1, 3, 5 µM) | SD rats P-MCAO i.p. 0.5 h before P-MCAO 10 mg/kg | Alleviated intracellular calcium overload Anti-apoptosis | ↑Hippocalcin ↓Caspase-3 ↓Bax ↑Bcl-2 | [110] | ||
| Hippocampal slices and neuron/glia co-culture Oxygen-glucose deprivation/reoxygenation (OGD/R) 0, 10 μM | SD rats T-MCAO i.p. 21 days before T-MCAO 25 mg/kg | Alleviated neurological deficits, brain infarction, and BBB disruption | ↑p-ERK ↑p-Akt ↓Protein phosphatase | [111] | ||
| NA | SD rats P-MCAO i.p. 0.5 h before P-MCAO 10 mg/kg | Anti-apoptosis Increased neuronal activity | ↑Parvalbumin | [112] | ||
| NA | SD rats P-MCAO i.p. 1 h before P-MCAO 30 mg/kg | Anti-apoptosis | ↓PARP ↓Caspase-3 | [113] | ||
| NA | SD rats P-MCAO i.p. 0.5 h before P-MCAO 10 mg/kg | Downregulated glutamate toxicity | ↑PP2A subunit B | [114] | ||
| BV2 cells OGD/R 20, 40 μM | ICR mice Hypoxic-ischemic brain injury i.p. for 2 days after injury 50 mg/kg | Anti-inflammatory Mitigated cognitive and motor function deficits | ↓TLR4/MyD88/NF-κB | [115] | ||
| NA | SD rats P-MCAO i.p. 1 h before p-MCAO 50 mg/kg | Improved energy metabolism | ↑γ-Enolase | [116] | ||
| Isoquercetin |  | Primary hippocampal neurons OGD/R 20, 40, 80 μg/mL | SD rats T-MCAO i.g. for 3 days after T-MCAO 5, 10, 20 mg/kg | Reduced infarct size Anti-apoptosis | ↓TLR4-NF-κB ↓p-JNK1/2 ↓p-p38 MAPK | [117] |
| NA | SD rats T-MCAO i.g. for 3 days before T-MCAO 5, 10, 20 mg/kg | Attenuated oxidative stress Anti-apoptosis | ↑Nrf2 ↓NOX4/ROS/NF-κB | [118] | ||
| Rutin |  | NA | Ovariectomized (OVX) SD rats T-MCAO i.p. for 5 days before T-MCAO 100 mg/kg | Decreased infarct size Attenuated neuron loss Improved sensorimotor performance and recognition memory | ↑BDNF-TrκB ↑NGF-TrkA | [119] |
| Kaempferol |  | NA | SD rats T-MCAO i.g. for 7 days after T-MCAO 25, 50, 100 mg/kg | Anti-inflammatory Attenuated BBB dysfunction | ↓p-p65 ↓MMP3 | [120] |
| NA | SD rats T-MCAO i.g. for 7 days before T-MCAO 0.5, 1, 2 mg/kg | Reduced infarct volume Anti-apoptosis | ↑p-Akt ↑Nrf-2 ↓p-NF-κB | [121] | ||
| PC12 cell line OGD/R 5, 10, 20 μM | NA | Ameliorated OGD-induced mitochondrial dysfunction | ↑Sirt1 ↓p66shc | [122] | ||
| Primary cortical neurons OGD 10 μM | C57BL/6 mice T-MCAO i.g. for 7 days before T-MCAO 50, 100, 200 mg/kg | Prevented HK-II detachment from mitochondria Ameliorated mitochondrial dysfunction | ↑p-Akt ↓Drp1 | [123] | ||
| Primary cortical neurons OGD/R 10 μM | NA | Decreased neuronal ferroptosis | ↑Nrf2/SLC7A11/GPx4 | [124] | ||
| Icariin |  | NA | SD rats T-MCAO i.g. for 28 days after T-MCAO 60 mg/kg | Promoted angiogenesis and neurogenesis | ↑PI3K/ERK1/2 ↑VEGF ↑BDNF | [125] |
| Primary microglia OGD/R 0.37, 0.74, 1.48 μM | NA | Decreased ER stress Anti-inflammatory | ↓IRE1/XBP1s | [126] | ||
| NA | SD rats T-MCAO i.g. for 28 days before T-MCAO 60 mg/kg | Promoted mild hypothermia-induced neuroprotection | ↑PPARs/Nrf2 ↓JAK2/STAT3/NF-κB | [127] | ||
| Myricetin |  | NA | SD rats P-MCAO i.g. for 7 days before T-MCAO 25 mg/kg | Reduced infarct volume Anti-apoptosis | ↓p-p38 MAPK ↓p-NF-κB ↑p-Akt | [128] |
| Human brain microvessel endothelial cells (HBMECs) OGD/R 10, 30, 60 μM | NA | Decreased enhancement of endothelial permeability Anti-inflammatory | ↑eNOS ↑p-Akt | [129] | ||
| Isoflavones | ||||||
| Puerarin |  | NA | SD rats T-MCAO i.p. 2 h before T-MCAO 50, 100 mg/kg | Alleviated neurological deficits Anti-apoptosis | ↑p-Akt1/p-GSK-3β/MCL-1 | [130] |
| NA | SD rats T-MCAO i.g. for 14 days before T-MCAO 50, 100 mg/kg | Suppressed excessive autophagy | ↓AMPK ↓ps317-ULK1 ↑mTOR ↑ps757-ULK1 | [131] | ||
| Genistein |  | N9/HT22 co-culture Primary microglia/primary cortical neuron co-culture OGD/R 5 μg/mL | C57BL/6J mice T-MCAO i.p. for 14 days before T-MCAO 10 mg/kg | Anti-inflammatory Anti-apoptosis | ↓NLRP3 | [132] |
| NA | OVX SD rats T-MCAO i.p. for 14 days before T-MCAO 10 mg/kg | Anti-oxidative stress Anti-apoptosis | ↑Nrf2 ↑NQO1 ↓Caspase-3 | [133] | ||
| NA | OVX SD rats T-MCAO i.p. for 14 days before T-MCAO 10 mg/kg | Anti-apoptosis | ↑PI3K-Akt-mTOR | [134] | ||
| Daidzein |  | NA | ICR mice T-MCAO i.p. 10 min after T-MCAO 10, 20, 30 mg/kg | Alleviated neuron impairment Anti-apoptosis | ↑PI3K/Akt/mTOR ↑BDNF/CREB | [135] |
| Flavones | ||||||
| Baicalein |  | NA | SD rats T-MCAO i.p. for 7 days after T-MCAO 200 mg/kg | Promoted M2 polarization of microglia Suppressed excessive autophagy | ↓MAPK/NF-κB ↑PI3K/Akt/mTOR | [136] |
| SH-SY5Y cell line OGD/R 0.1–8 μM | SD rats T-MCAO intravenous injection (i.v.) just before refusion 2.5, 5, 10 mg/kg | Anti-oxidative stress Anti-inflammatory | ↑Nrf2 ↓NF-κB ↓LOX-1 ↓AMPK | [137] | ||
| BV2 cell line LPS/IFNr stimulation or OGD/R 45 μM | C57BL/6J mice T-MCAO i.g. for 3 days after T-MCAO 100 mg/kg | Anti-inflammatory Promoted M2 polarization of microglia | ↓TLR4/NF-κB ↓p-STAT1 | [138] | ||
| SH-SY5Y cell line OGD/R 1, 5, 10, 15, 20 μM | SD rats T-MCAO i.g. for 7 days after T-MCAO 100 mg/kg | Anti-apoptosis Reduced infarct volume | ↓PARP-1 ↓Nuclear translocation of MIF and AIF | [139] | ||
| PC12 cell line OGD/R 0.02, 0.1, 0.5 µM | SD rats T-MCAO i.g. for 7 days after T-MCAO 100 mg/kg | Anti-oxidative stress Anti-apoptosis | ↓Calpain 1 ↓Nuclear translocation of AIF | [140] | ||
| Baicalin |  | Primary astrocytes OGD/R 1, 10 μM | SD T-MCAO i.p. 0.5 h before refusion 50 mg/kg | Anti-excitotoxic | ↓SDH ↑GS | [141] |
| Scutellarin |  | BV2 cell line LPS stimulation 0.54 μM | SD rats P-MCAO i.p. 2 h before MCAO and 12, 24, 36, 48, 60 h after MCAO 100 mg/kg | Decreased microglial activation Anti-inflammatory | ↓p-p38 MAPK ↓p-JNK | [142] |
| NA | SD rats P-MCAO i.p. for 7 days before P-MCAO 20, 50, 100 mg/kg | Alleviated cognitive impairments Anti-inflammatory | ↓PARP-1/NF-κB | [143] | ||
| Primary astrocytes OGD/R 10, 50 μM | SD rats T-MCAO i.p. 2 h before MCAO and 12, 24, 36, 48, 60 h after MCAO 50, 100 mg/kg | Anti-oxidative stress | ↓NOX2 ↑Cx43 | [144] | ||
| NA | SD rats T-MCAO i.v. for 7 days after T-MCAO 0.33 mg/kg | Suppressed excessive autophagy | ↓LC3-II/LC3-I | [145] | ||
| Luteolin |  | NA | SD rats T-MCAO i.p. 0, 12 h after T-MCAO 20, 40, 80 mg/kg | Alleviated neurologic deficits and cerebral edema Anti-inflammatory | ↑Nrf2 ↓PPARγ/NF-κB | [146] |
| NA | SD rats T-MCAO i.p. for 7 days after T-MCAO 15, 30, 60 mg/kg | Suppressed excessive autophagy Ameliorated mitochondrial dysfunction | ↑Sirt3/AMPK/mTOR | [147] | ||
| Chrysin |  | NA | Wistar rats T-MCAO i.g. for 21 days before T-MCAO 10, 30, 100 mg/kg | Prevented cognitive and hippocampal LTP impairments | ↓IL-1β ↓TNF-α | [148] |
| SH-SY5Y cell line OGD/R 10, 20, 40, 80, 160, 320 μM | SD rats T-MCAO i.g. for 7 days after T-MCAO 10, 20 mg/kg | Anti-inflammatory Anti-apoptosis | ↑PI3K/Akt/mTOR | [149] | ||
| NA | SD rats T-MCAO i.g. for 14 days before T-MCAO 30 mg/kg | Anti-inflammatory | ↓iNOS ↓TNF-α | [150] | ||
| Apigenin |  | HBMEC OGD/R 2.5, 5 μM | SD rats T-MCAO i.p. for 14 days after T-MCAO 25 mg/kg | Suppressed excessive autophagy Promoted neovascularization | ↑Caveolin-1 ↑VEGF | [151] |
| PC12 cell line 1.2 mM CoCl2 stimulation 0–200 µg/mL | SD rats T-MCAO i.p. for 7 days after T-MCAO 25 mg/kg | Ameliorated mitochondrial dysfunction | ↓ROS | [152] | ||
| Flavanols | ||||||
| (-)-Epigallocatechin gallate |  | NA | SD rats T-MCAO i.p. immediately after T-MCAO 20 mg/kg | Anti-apoptosis | ↑PI3K/Akt/eNOS | [153] |
| Neurosphere culture 10 ng/mL LPS stimulation 10, 20, 40 μM for 7 days | C57BL/6 mice T-MCAO Injection into left ventricle for 14 days starting at 14 days post injury 2 μg | Promoted the M2 polarization of microglia Promoted neurogenesis | ↑PI3K/Akt | [154] | ||
| NA | SD rats P-MCAO i.p. just before P-MCAO 50 mg/kg | Anti-apoptosis | ↓Caspase-3 ↓PARP | [155] | ||
| HT22 cell line Glutamate stimulation 10, 20, 40 μM | SD rats P-MCAO i.p. just before P-MCAO 50 mg/kg | Alleviated neurological deficits Suppressed glutamate-induced oxidative stress | ↑Thioredoxin/ASK-1 | [156] | ||
| (-)-Epicatechin gallate |  | HBMEC OGD/R 0.5, 1, 2, 4 μM | NA | Promoted neovascularization Alleviated apoptosis and autophagy | ↑VEGF ↓LC3-I/II ↑mTOR ↑Bcl-2/Bax | [157] |
| Procyanidin |  | BV2 cell line OGD 10 μM | SD rats T-MCAO i.p. 1 h before P-MCAO 20, 40, 80 mg/kg | Ameliorated neurological deficits Anti-inflammatory | ↓TLR4-p38-NF-κB-NLRP3 | [158] |
| Flavanones | ||||||
| Naringenin |  | Primary cortical neuron OGD/R 20, 40, 80 μM | SD rats T-MCAO i.p. immediately before T-MCAO 20 mg/kg | Anti-oxidative stress | ↑Nrf2 | [159] |
| Naringin |  | SH-SY5Y cell line OGD/R 100 μM/200 μM | SD rats T-MCAO i.v. immediately before T-MCAO 80, 120, 160 mg/kg | Inhibited mitophagy | ↓ONOO− ↓3-NT ↓LC3-II/LC3-I | [160] |
| Hesperetin |  | BV2 cell line OGD/R 100 μM | C57BL/6 mice T-MCAO i.p. for 7 days after T-MCAO 30 mg/kg | Anti-inflammatory Promoted the M2 polarization of microglia | ↓TLR4/NF-κB | [161] |
| Ginkgetin aglycone | - | NA | Wistar rats T-MCAO i.p. for 5 days before T-MCAO 100, 200 mg/kg | Anti-oxidative stress Anti-inflammatory | ↓JAK2/STAT3/Sirt1 | [162] |
| Anthocyanins | ||||||
| Cyanidin-3-glucoside |  | NA | ICR mice T-MCAO i.g. for 7 days before T-MCAO 100, 150, 200 mg/kg | Anti-inflammatory | ↓TLR4/NF-κB ↓NLRP3 | [163] |
| HT22 cell line Glutamate stimulation 0.05–1 μM | NA | Anti-oxidative stress induced-ER stress | ↑ERK/Nrf2 ↑Antioxidant enzyme | [164] | ||
| Petunidin-3-O-rutinoside (p-coumaroyl)-5-O-glucoside | - | NA | SD rats T-MCAO i.p. for 7 days before T-MCAO 50, 100, 200 mg/kg | Protected neurovascular unit | ↓NLRP3 ↓NF-κB ↓MMP9 | [165] |
| SH-SY5Y cell line OGD/R 10, 100, 1000 μg/mL | NA | Enhanced autophagic flux | ↓SQSTM1 ↑LC3B II/LC3B I | [166] | ||
| Polyphenolic Compound | Chemical Structure | Models and Treatments | Observed Effects | Mechanisms | Reference | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| Cinnamic Acid Derivatives | ||||||
| Ferulic acid |  | BMEC OGD/R 100, 200, 300, 400 μM | NA | Increased punctate-mitochondria-dependent mitophagy | ↑LC3-II | [192] |
| NA | SD rats P-MCAO i.v. immediately after P-MCAO 60, 80, 100 mg/kg | Anti-apoptosis | ↑Akt/mTOR/4E-BP1/Bcl-2 | [193] | ||
| NA | SD rats P-MCAO i.v. 0.5 h after P-MCAO 80, 100 mg/kg | Anti-apoptosis Promoted autophagy | ↑HSP70/Bcl-2 ↑Beclin 1 | [194] | ||
| Rosmarinic acid |  | NA | CD-1 mice T-MCAO i.p. immediately after T-MCAO 10, 20, 40 mg/kg | Anti-oxidative stress Anti-apoptosis | ↑Akt/Nrf2/HO-1 | [195] |
| Chlorogenic acid |  | NA | Wistar rats T-MCAO i.g. for 3 days before T-MCAO 15, 30, 60 mg/kg | Decreased mortality Reduced infarct volume | ↓ICAM-1 ↓VCAM-1 ↑EPO ↑HIF-1α ↑NGF | [196] |
| NA | SD rats P-MCAO i.p. 2 h after P-MCAO 30 mg/kg | Alleviated neurobehavioral symptoms Anti-apoptosis | ↓Caspase-3 ↓Caspase-7 ↓PARP | [197] | ||
| NA | Wistar rats T-MCAO i.p. 10 min before T-MCAO and 10 min after refusion 10 mg/kg | Anti-apoptosis | ↑miR-23b ↓TAB3/NF-κB/p53 | [198] | ||
| NA | SD rats T-MCAO i.p. 7 days before T-MCAO 20, 100, 500 mg/kg | Anti-oxidative stress | ↑Nrf2/NQO-1/HO-1 | [199] | ||
| NA | SD rats P-MCAO i.p. 2 h after P-MCAO 30 mg/kg | Anti-inflammatory Inhibited the activation of astrocytes and microglia | ↓NF-κB | [200] | ||
| Salvianolic acid A |  | NA | SD rats T-MCAO i.p. immediately after T-MCAO 5, 10, 20 mg/kg | Protect BBB Anti-inflammatory | ↓MMP9 ↓NF-κB p65 | [201] |
| SH-SY5Y cell line OGD/R 0.05, 0.5, 5 μM | SD rats T-MCAO i.v. immediately after T-MCAO 5, 10, 20 mg/kg | Improved neurological function Anti-apoptosis | ↑Akt/FOXO3a ↓BIM/Caspase-3 | [202] | ||
| PC12 cell line OGD/R 5 μM | SD rats T-MCAO i.p. 0, 6 h after T-MCAO 20 mg/kg | Anti-apoptosis Anti-inflammatory | ↑miR-499a ↑Wnt3a/β-catenin ↓DDK1 | [203] | ||
| HBMEC OGD 1, 3, 10 μM | SD rats Autologous thrombus stroke model i.g. for 5 days before stroke 10 mg/kg | Alleviated intracerebral hemorrhage Suppressed vascular endothelial dysfunction | ↓VEGFA-Src-VAV2-Rac1-PAK | [204] | ||
| NA | SD rats T-MCAO i.p. 15 min before T-MCAO 5, 10 mg/kg | Anti-inflammatory | ↓TLRs/MyD88 | [205] | ||
| NA | SD rats autologous thrombus stroke model i.p. for 14 days after stroke 10 mg/kg | Promoted endogenous neurogenesis | ↑Wnt3a/β-catenin ↓GSK-3β | [206] | ||
| Salvianolic acid B |  | NA | Wistar rats T-MCAO i.p. for 3 days before T-MCAO 3, 6, 12 mg/kg | Inhibited platelet activation Anti-inflammatory | ↓P-selection ↓CD40L ↓CD40/NF-κB | [207] |
| Primary astrocytes/primary cortical neurons OGD/R 800 ng/mL | C57BL/6J mice T-MCAO i.p. immediately after refusion 12 mg/kg | Promoted glycogenolysis Anti-oxidative stress | ↑GP activity ↑NADPH ↑GSH | [208] | ||
| Benzoic acid derivatives | ||||||
| Protocatechuic acid |  | NA | Wistar rats T-MCAO i.p. 1.5 h after T-MCAO 10, 30, 50 mg/kg | Anti-apoptosis Inhibited microglial activation | ↑CREB ↓Caspase-3 | [209] |
| Gallic acid |  | NA | C57BL/6J mice T-MCAO i.p. 1, 12, 24, 48, 72 h after T-MCAO 50, 100, 200 mg/kg | Inhibited microglial M1 polarization Protect BBB | ↓MMP9 | [210] |
| Vanillic acid |  | NA | Wistar rats Transient bilateral common carotid artery occlusion and reperfusion (TBCCAO/R) i.g. for 14 days before TBCCAO/R 100 mg/kg | Restored spatial memory Anti-inflammatory | ↓IL-6 ↓TNF-α ↑IL-10 | [211] |
| Rhein |  | NA | SD rats T-MCAO i.g. for 3 days after T-MCAO 25, 50, 100 mg/kg | Anti-oxidative stress Anti-apoptosis | ↑SOD ↑Bcl-2 ↓Bax ↓Caspase-3 ↓Caspase-9 | [212] |
| Lignan | Chemical Structure | Models and Treatments | Observed Effects | Mechanisms | Reference | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| Magnolol |  | BMEC OGD/R 1, 10 μM Primary microglia LPS stimulation 0.01, 0.1, 1, 10 μM | Kunming mice T-MCAO i.v. 0, 1, 2 h after T-MCAO 1.4, 7.0, 35.0 μg/kg | Protect BBB Anti-inflammatory | ↓p-EphA2 ↑ZO-1 ↑Occludin ↓TNF-α | [226] |
| NA | SD rats T-MCAO i.p. immediately after T-MCAO 25 mg/kg | Anti-inflammatory Anti-apoptosis | ↑Sirt1 ↑Bcl-2 ↓Bax ↓Ac-FOXO1 ↓TNF-α | [227] | ||
| BV2/RAW264.7 cell line LPS stimulation 0.1–50 μM | SD rats T-MCAO i.p. 30 min before T-MCAO/2 h after T-MCAO 0.01, 0.1, 1 mg/kg | Anti-oxidative stress Anti-inflammatory | ↓TNF-α ↓IL-6 ↓NOX | [228] | ||
| NA | SD rats T-MCAO i.p. for 7 days before T-MCAO 75 mg/kg | Anti-apoptosis | ↑BDNF ↓Bax | [229] | ||
| Schisandrin A |  | Neural progenitor cell line/primary cortical neurons 0.1, 1, 10 μM | C57/BL6 mice photothrombosis model i.p. 21 days after infarction 12 mg/kg | Promoted neural cell proliferation and differentiation | ↑Cdc42 ↑GTPase | [230] |
| Schisandrin B |  | NA | SD rats T-MCAO i.v. for 3 days before T-MCAO 15, 30 mg/kg | Anti-inflammatory | ↓TLR4/NF-κB | [231] |
| Sesamol |  | NA | SD rats T-MCAO i.p. for 7 days before T-MCAO 25 mg/kg | Anti-apoptosis | ↑Bcl-2 ↓Bax ↓Caspase-3 | [232] |
| Arctigenin |  | Primary cortical neurons OGD/R 100 ng/mL | SD rats T-MCAO i.p. for 3 days before T-MCAO 20 mg/kg | Anti-inflammatory | ↑Sirt1 ↓NLRP3 | [233] |
| Stilbene | Chemical Structure | Models and Treatments | Observed Effects | Mechanisms | Reference | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| Resveratrol |  | Primary microglia OGD/R 5 μM | C57BL/6 mice T-MCAO i.g. for 3 days after T-MCAO 200 mg/kg | Inhibited pro-inflammatory microglia activation | ↓CD147/MMP9 | [236] |
| NA | SD rats T-MCAO i.p. for 7 days after T-MCAO 20 mg/kg | Alleviated cognitive impairment Reduced neuronal loss | ↓JAK/ERK/STAT | [237] | ||
| NA | SD rats P-MCAO i.p. 2/12 h after P-MCAO 100 mg/kg | Reduced neurological deficits and cerebral water content | ↑PI3K/Akt | [238] | ||
| NA | SD rats T-MCAO i.p. for 7 days before T-MCAO 30 mg/kg | Improved neurological function Reduced infarct size | ↑p-JAK2/p-STAT3 ↑PI3K/Akt/mTOR | [239] | ||
| NA | C57BL/6 mice T-MCAO i.p. for 3 days after T-MCAO 200 mg/kg | Modulated the gut-brain axis | ↑IL-4/Th2 ↑IL-10/Treg | [240] | ||
| NA | Swiss albino mice T-MCAO i.p. 5 min before refusion 30 mg/kg | Anti-oxidative stress Reduced AChE activity | ↑Sirt1 | [241] | ||
| Primary cortical neurons Glutamate stimulated excitotoxicity 0.004–400 μM | Wistar rats T-MCAO i.v. immediately after T-MCAO 1.8 mg/kg | Promoted autophagy | ↑p-AMPK ↑Beclin 1 | [242] | ||
| NA | SD rats T-MCAO i.p. 30 min before refusion 20 mg/kg | Anti-apoptosis | ↑Sirt1/miR-149–5p/p53 | [243] | ||
| NA | C57/BL mice T-MCAO i.p. 0, 8, 18 h after T-MCAO 100 mg/kg | Promoted the M2 polarization of microglia | ↓miR-155 | [244] | ||
| N2a cell line OGD/R 10, 20 μM | NA | Modulated mitochondrial homeostasis | ↑p-AMPK-Mfn1 | [245] | ||
| Primary cortical neurons OGD/R 5 μM | SD rats T-MCAO i.p. for 7 days before T-MCAO 30 mg/kg | Promoted nerve regeneration | ↑Sonic hedgehog | [246] | ||
| Pterostilbene |  | NA | Wistar rats T-MCAO i.g. for 30 days before T-MCAO 35 mg/kg | Inhibited inflammatory cell infiltration Anti-inflammatory | ↓COX-2 ↓PGE2 ↓NF-κB | [247] |
| BV2 cell line LPS stimulation 1, 10 μM | SD rats T-MCAO i.p. immediately after refusion 7, 14, 28 mg/kg | Anti-oxidative stress Anti-inflammatory | ↓NADPH ↓NF-κB | [248] | ||
| HT22/U251 co-culture OGD/R 2.5, 5 μM | SD rats T-MCAO i.p. 1 h after T-MCAO 7, 14, 28 mg/kg | Anti-inflammatory | ↓NF-κB | [249] | ||
| Piceatannol |  | NA | C57BL/6 mice T-MCAO i.g. for 6 days after T-MCAO 10,20 mg/kg | Anti-oxidative stress Anti-apoptosis | ↑Sirt1/FoxO1 | [250] |
| PC12 cell line OGD/R 2.5, 10, 40, 160 μM | ICR mice T-MCAO i.p. Immediately after refusion 5, 10, 20 mg/kg | Anti-oxidative stress | ↑Nrf2/HO-1 | [251] | ||
| Polydatin |  | HUVEC/BMEC OGD 20 μM | SD rats T-MCAO i.v. 10 min before T-MCAO 30 mg/kg | Protected cerebrovascular endothelial cells and enhanced BBB integrity | ↑C/EBPβ/MALAT1/CREB/PGC-1α/PPARγ | [252] |
| Chemical Structure | Models and Treatments | Observed Effects | Mechanisms | Reference | ||
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| Curcumin |  | SH-SY5Y cell line OGD/R 25 μM | NA | Anti-oxidative stress | ↑miR-1287–5p ↓LONP2 | [258] |
| Primary microglia LPS stimulation 12.5 μM | C57BL/6J mice T-MCAO i.p. for 7 days after T-MCAO 150 mg/kg | Inhibited pyroptosis | ↓NLRP3 ↓GSDMD-N | [259] | ||
| PC12 cell line OGD/R 20 μM | NA | Anti-inflammatory | ↓CCL3 ↓TLR4/MyD88/MAPK/NF-κB | [260] | ||
| Primary cortical neurons OGD/R 5 μM | SD rats T-MCAO i.p. immediately after refusion 100 mg/kg | Preserved mitochondrial function Enhanced mitophagy | ↑LC3-II/LC3-I | [261] | ||
| PC12 cell line OGD/R 20 μM | SD rats T-MCAO i.p. 24 and 1 h before T-MCAO 100 mg/kg | Decreased intracellular calcium ion concentration BBB protection | ↑PKC-θ ↓ORai1 | [262] | ||
| NA | SD rats T-MCAO i.p. 30 min before T-MCAO 300 mg/kg | Anti-inflammatory BBB protection | ↓NF-κB ↓MMP9 | [263] | ||
| BV2 cell line LPS stimulation 12.5, 25 μM | C57BL/6 mice P-MCAO i.p. 0 and 24 h after T-MCAO 150 mg/kg | Promoted microglial M2 polarization | ↓TNF-α ↓iNOS ↑CD206 | [264] | ||
| NA | Wistar rats T-MCAO i.p. 30 min before T-MCAO 300 mg/kg | Reduced neurological dysfunction Anti-inflammatory Anti-apoptosis | ↓ICAM-1 ↓MMP9 ↓Caspase-3 ↓NF-κB | [265] | ||
| NA | Wistar rats T-MCAO i.p. 2 h before T-MCAO 150 mg/kg | Inhibited ER stress | ↓GADD153 ↓Caspase-12 | [266] | ||
| NA | SD rats T-MCAO i.p. 30 min after T-MCAO 200 mg/kg | Anti-inflammatory Suppressed excessive autophagy | ↑PI3K/Akt/mTOR ↓TLR4/p38 MAPK | [267] | ||
| Primary astrocytes OGD/R 5, 10, 20 μM | SD rats T-MCAO i.p. 30 min before T-MCAO 300 mg/kg | Decreased infarct size Anti-apoptosis | ↑MEK/ERK/CREB | [268] | ||
| PC12 cell line OGD/R 10, 20 μM | SD rats T-MCAO i.g. for 3 days after T-MCAO 100, 300 mg/kg | Anti-oxidative stress Anti-inflammatory Anti-apoptosis | ↑miR-7–5p ↓RelA p65 | [269] | ||
| PC12 cell line OGD/R 5 μM | NA | Suppressed excessive autophagy | ↓HIF-1α ↓LC3-II ↓p62 | [270] | ||
| N2A cell line OGD/R 5, 15, 25, 35 μM | C57BL/6 mice T-MCAO i.p. 1 h after T-MCAO 100, 200, 300, 400 mg/kg | Anti-apoptosis Ameliorated mitochondrial dysfunction | ↑Bcl-2 ↓Bax ↓Caspase-3 | [271] | ||
| HT22 cell line OGD/R 100 ng/mL | NA | Anti-oxidative stress | ↑SOD2 | [272] | ||
| NA | Diabetic SD rats T-MCAO i.g. after T-MCAO 40 mg/kg | Decreased infarct size Regulated glucose uptake Anti-apoptosis | ↑GLUT1 ↑GLUT3 | [273] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Lin, F.; Wang, J.; Pan, X.; Sun, L.; Wu, W. Polyphenols for the Treatment of Ischemic Stroke: New Applications and Insights. Molecules 2022, 27, 4181. https://doi.org/10.3390/molecules27134181
Liu S, Lin F, Wang J, Pan X, Sun L, Wu W. Polyphenols for the Treatment of Ischemic Stroke: New Applications and Insights. Molecules. 2022; 27(13):4181. https://doi.org/10.3390/molecules27134181
Chicago/Turabian StyleLiu, Shuhan, Feng Lin, Jian Wang, Xiaoqiang Pan, Liguang Sun, and Wei Wu. 2022. "Polyphenols for the Treatment of Ischemic Stroke: New Applications and Insights" Molecules 27, no. 13: 4181. https://doi.org/10.3390/molecules27134181