Effects of Frankincense Compounds on Infection, Inflammation, and Oral Health
Abstract
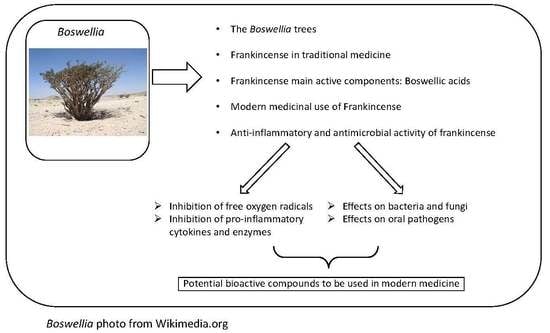
:1. Introduction
2. Description of Frankincense Resins, Oils, and Boswellic Acids
3. Modern Medicinal Uses of Frankincense
3.1. Anti-Cancer
3.2. Hypolipidemic and Hypoglycemic Effects
4. Antimicrobial Activity of Frankincense
4.1. Activity of Boswellia against Microbes
4.2. Activity of Boswellia against Fungal and Bacterial Biofilms
4.3. Antimicrobial Activity against Oral Pathogens
5. Anti-Inflammatory Effects of Frankincense
6. Concluding Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.; Ryu, K.; Zhi, S.; Otto, S.J.G.; Neumann, N.F. Naturalized Escherichia coli in Wastewater and the Co-evolution of Bacterial Resistance to Water Treatment and Antibiotics. Front. Microbiol. 2022, 13, 810312. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense—Therapeutic properties. Postepy Hig. Med. Dosw. (Online) 2016, 70, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015, 176, 118–134. [Google Scholar] [CrossRef]
- Eslamieh, J. Cultivation of Boswellia, 2nd ed.; A Book’s Mind: Fort Collins, CO, USA, 2017. [Google Scholar]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef]
- Britannica, The Editors of Encyclopaedia. Frankincense. Available online: https://www.britannica.com/topic/frankincense (accessed on 21 February 2022).
- Bokelmann, J.M. Medicinal Herbs in Primary Care: An Evidence-Guided Reference for Healthcare Providers. In Frankincense/Boswellia (Boswellia serrata/sacra/carterii): Bark Resin; Bokelmann, J.M., Ed.; Medicinal Herbs in Primary Case; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–360. [Google Scholar]
- The World Flora Online. 2022. Available online: http://www.worldfloraonline.org/ (accessed on 21 May 2022).
- Al-Harrasi, A.; Khan, A.L.; Asaf, S.; Al-Rawahi, A. Taxonomy, Distribution and Ecology of Boswellia. In Biology of Genus Boswellia; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Shah, S.A.; Rathod, I.S.; Suhagia, B.N.; Pandya, S.S.; Parmar, V.K. A simple high-performance liquid chromatographic method for the estimation of boswellic acids from the market formulations containing Boswellia serrata extract. J. Chromatogr. Sci. 2008, 46, 735–738. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, M.R.; Ryan, A.E.; Lee, D.Y.; Zhongze, M.; Wu-Yan, Z.; Via, C.S. Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro. Clin. Diagn. Lab. Immunol. 2005, 12, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J.R.; Langenheim, J.H. Plant resins: Chemistry, evolution, ecology and ethnobotany. Ann. Bot. 2004, 93, 2. [Google Scholar] [CrossRef] [Green Version]
- Hughes, L. The Funerals of the Russian Emperors and Empresses. In Monarchy and Religion: The Transformation of Royal Culture in Eighteenth-Century Europe; Schaich, M., Ed.; Oxford University Press: Oxford, UK, 2007; pp. 395–419. [Google Scholar]
- Berhanu, Y.; Vedeld, P.; Angassa, A.; Aune, J.B. The contribution of frankincense to the agro-pastoral household economy and its potential for commercialization—A case from Borana, southern Ethiopia. J. Arid Environ. 2020, 186, 104423. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- The Word Flora Online. Boswellia sacra Flueck. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569724 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia serrata Roxb. Ex Colebr. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569726 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia odorata Hutch. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569716 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia popoviana Hepper. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569721 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia ruspoliana Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569723 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia socotrana Balf.f. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569727 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia holstii Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569705 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia microphylla Chiov. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569709 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia multifoliolata Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569710 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia nana Hepper. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569711 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia ogadensis Vollesen. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569717 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia ovalifoliolata N.P.Balakr. & A.N.Henry. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569718 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia pirottae Chiov. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569720 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia ameero Balf.f. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569684 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia boranensis Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569688 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia bricchettii (Chiov.) Chiov. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569689 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia bullata Thulin. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569690 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia chariensis Guillaumin. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569695 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia dioscoridis Thulin. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569697 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia elongata Balf.f. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569699 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia frereana Birdw. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569700 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia globosa Thulin. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000506703 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia dalzielii Hutch. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569696 (accessed on 21 May 2022).
- The Word Flora Online. Ambilobea madagascariensis (Capuron) Thulin, Beier & Razafim. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000741637 (accessed on 21 May 2022).
- The Word Flora Online. Garuga floribunda Decne. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000694779 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia papyrifera (Caill. Ex Delile) Hochst. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569719 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia rivae Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569722 (accessed on 21 May 2022).
- The Word Flora Online. Boswellia hildebrandtii Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569702 (accessed on 14 June 2022).
- The Word Flora Online. Boswellia elegans Engl. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569698 (accessed on 17 June 2022).
- The Word Flora Online. Boswellia neglecta S.Moore. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000569712 (accessed on 21 May 2022).
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Jones, J.K.N.; Nunn, J.R. The Structure of Frankincense Gum. J. Am. Chem. Soc. 1955, 77, 5745–5746. [Google Scholar] [CrossRef]
- Hosain, N.A.; Ghosh, R.; Bryant, D.L.; Arivett, B.A.; Farone, A.L.; Kline, P.C. Isolation, structure elucidation, and immunostimulatory activity of polysaccharide fractions from Boswellia carterii frankincense resin. Int. J. Biol. Macromol. 2019, 133, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Amri, A.S.L.; Jesil, A.; Salim, A.; Saravanam, A.M. Extraction of Essential Oil from Frankincense Using Steam Distillation. Int. J. Trend Res. Dev. 2019, 6, 3. [Google Scholar]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.L.; Cui, H.H.; Hua, H.M.; Jing, Y.K.; Liang, S.W. Two new triterpenoids from the resin of Boswellia carterii. J. Asian Nat. Prod. Res. 2011, 13, 193–197. [Google Scholar] [CrossRef]
- Ren, P.; Ren, X.; Cheng, L.; Xu, L. Frankincense, pine needle and geranium essential oils suppress tumor progression through the regulation of the AMPK/mTOR pathway in breast cancer. Oncol. Rep. 2018, 39, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Lou, H.X. Bioactive constituents of myrrh and frankincense, two simultaneously prescribed gum resins in chinese traditional medicine. Chem. Biodivers. 2008, 5, 540–553. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Singh, S.; Velaga, S. Boswellic acid—Medicinal use of an ancient herbal remedy. J. Herb. Med. 2016, 6, 8. [Google Scholar] [CrossRef]
- Chiavari, G.; Galletti, G.C.; Piccaglia, R.; Mohamud, M.A. Differentiation between Resins Boswellia carterii and Boswellia frereana (Frankincense) of Somali Origin. J. Essent. Oil Res. 1990, 3, 2. [Google Scholar] [CrossRef]
- Wahab, S.M.; Aboutabl, E.A.; El-Zalabani, S.M.; Fouad, H.A.; De Pooter, H.L.; El-Fallaha, B. The essential oil of olibanum. Planta Med. 1987, 53, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, Y.; Liu, L.; Ling, D.; Qin, X.; Tian, J. Analysis of the Chemical Constituents of Essential Oil of Boswellia carterii Birdwood from Somali. Chin. J. Pharm. Anal. 1993, 13, 3. [Google Scholar]
- Mohamed, A.A.; Ali, S.I.; Kabiel, H.F.; Hegazy, A.K.; Kord, M.A.; EL-Baz, F.K. Assessment of Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Boswellia carterii Resin. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 8. [Google Scholar]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Mikhaeil, B.R.; Maatooq, G.T.; Badria, F.A.; Amer, M.M. Chemistry and immunomodulatory activity of frankincense oil. Z. Nat. C 2003, 58, 230–238. [Google Scholar] [CrossRef]
- DeCarlo, A.; Johnson, S.; Okeke-Agulu, K.I.; Dosoky, N.S.; Wax, S.J.; Owolabi, M.S.; Setzer, W.N. Compositional analysis of the essential oil of Boswellia dalzielii frankincense from West Africa reveals two major chemotypes. Phytochemistry 2019, 164, 24–32. [Google Scholar] [CrossRef]
- Dimas Kubmarawa, I.A.O.; Okorie, D.A.; Olawore, N.O.; Kasali, A.A. Constituents of the Essential Oils of Boswellia dalzielii Hutch. from Nigeria. J. Essent. Oil Res. 2011, 18, 2. [Google Scholar] [CrossRef]
- Kohoude, M.J.; Gbaguidi, F.; Agbani, P.; Ayedoun, M.A.; Cazaux, S.; Bouajila, J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017, 55, 33–42. [Google Scholar] [CrossRef] [Green Version]
- De Rapper, S.; Van Vuuren, S.F.; Kamatou, G.P.; Viljoen, A.M.; Dagne, E. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils—A combination from the pharaonic pharmacopoeia. Lett. Appl. Microbiol. 2012, 54, 352–358. [Google Scholar] [CrossRef]
- Gupta, I.; Parihar, A.; Malhotra, P.; Gupta, S.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001, 67, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [Green Version]
- Iram, F.; Khan, S.A.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523. [Google Scholar] [CrossRef]
- Padhi, M.; Mahapatra, S. Boswellia serrata: A review of its traditional uses, phytochemistry and pharmacology. Int. Rev. Biophys. Chem. (IREBIC) 2013, 4, 74–83. [Google Scholar]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, F.; Capasso, F.; Capasso, R.; Ascione, V.; Aviello, G.; Longo, R.; Izzo, A.A. Effect of Boswellia serrata on intestinal motility in rodents: Inhibition of diarrhoea without constipation. Br. J. Pharmacol. 2006, 148, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Ammon, H.P. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef] [Green Version]
- Gupta, I.; Gupta, V.; Parihar, A.; Gupta, S.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with bronchial asthma: Results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res. 1998, 3, 511–514. [Google Scholar]
- Shah, B.A.; Qazi, G.N.; Taneja, S.C. Boswellic acids: A group of medicinally important compounds. Nat. Prod. Rep. 2009, 26, 72–89. [Google Scholar] [CrossRef]
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res. 1997, 2, 37–43. [Google Scholar]
- Banno, N.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Tabata, K.; Nakamura, Y.; Nishimura, R.; Kimura, Y.; Suzuki, T. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J. Ethnopharmacol. 2006, 107, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Langmead, L.; Rampton, D.S. Review article: Complementary and alternative therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 23, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Holtmeier, W.; Zeuzem, S.; Preiss, J.; Kruis, W.; Bohm, S.; Maaser, C.; Raedler, A.; Schmidt, C.; Schnitker, J.; Schwarz, J.; et al. Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn’s disease: Good safety profile but lack of efficacy. Inflamm. Bowel Dis. 2011, 17, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 2006, 13, 3359–3369. [Google Scholar] [CrossRef]
- Krieglstein, C.F.; Anthoni, C.; Rijcken, E.J.; Laukotter, M.; Spiegel, H.U.; Boden, S.E.; Schweizer, S.; Safayhi, H.; Senninger, N.; Schurmann, G. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal. Dis. 2001, 16, 88–95. [Google Scholar] [CrossRef]
- Moussaieff, A.; Mechoulam, R. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J. Pharm. Pharmacol. 2010, 61, 1281–1293. [Google Scholar] [CrossRef]
- Yu, G.; Xiang, W.; Zhang, T.; Zeng, L.; Yang, K.; Li, J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 225. [Google Scholar] [CrossRef]
- Huang, M.T.; Badmaev, V.; Ding, Y.; Liu, Y.; Xie, J.G.; Ho, C.T. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors 2000, 13, 225–230. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Q.; Sun, L. Five terpenoids from the gum resin of Boswellia carterii and their cytotoxicity. Fitoterapia 2021, 154, 105017. [Google Scholar] [CrossRef]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.M.; Shih, P.T.; Fang, Y.T.; Woolley, C.; Young, G.; Lin, H.K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Chen, D.; Han, R.; Fang, Q.; Waxman, S.; Jing, Y. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol. Cancer Ther. 2005, 4, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Casapullo, A.; Cassiano, C.; Capolupo, A.; Del Gaudio, F.; Esposito, R.; Tosco, A.; Riccio, R.; Monti, M.C. β-Boswellic acid, a bioactive substance used in food supplements, inhibits protein synthesis by targeting the ribosomal machinery. J. Mass Spectrom. 2016, 51, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarnejad, T.; Saidijam, M.; Moradkhani, S.; Najafi, R. Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Prostaglandins Other Lipid Mediat. 2017, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.S.; Singh, B.K.; Tripathi, Y.B. Extract of gum resins of Boswellia serrata L. inhibits lipopolysaccharide induced nitric oxide production in rat macrophages along with hypolipidemic property. Indian J. Exp. Biol. 2005, 43, 509–516. [Google Scholar] [PubMed]
- Ahangarpour, A.; Heidari, H.; Fatemeh, R.A.; Pakmehr, M.; Shahbazian, H.; Ahmadi, I.; Mombeini, Z.; Mehrangiz, B.H. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J. Diabetes Metab. Disord. 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrzadi, S.; Tavakolifar, B.; Huseini, H.F.; Mosavat, S.H.; Heydari, M. The Effects of Boswellia serrata Gum Resin on the Blood Glucose and Lipid Profile of Diabetic Patients: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Evid. Based Integr. Med. 2018, 23, 2515690 × 18772728. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, I.; Halim, S.A.; Rehman, N.U.; Karim, N.; Ahmad, W.; Khan, M.; Csuk, R.; Al-Harrasi, A. Anti-diabetic potential of beta-boswellic acid and 11-keto-beta-boswellic acid: Mechanistic insights from computational and biochemical approaches. Biomed. Pharmacother. 2022, 147, 112669. [Google Scholar] [CrossRef]
- Azemi, M.E.; Namjoyan, F.; Khodayar, M.J.; Ahmadpour, F.; Darvish Padok, A.; Panahi, M. The Antioxidant Capacity and Anti-diabetic Effect of Boswellia serrata Triana and Planch Aqueous Extract in Fertile Female Diabetic Rats and the Possible Effects on Reproduction and Histological Changes in the Liver and Kidneys. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Mahdian, D.; Abbaszadeh-Goudarzi, K.; Raoofi, A.; Dadashizadeh, G.; Abroudi, M.; Zarepour, E.; Hosseinzadeh, H. Effect of Boswellia species on the metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2020, 23, 1374–1381. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Hasson, S.S.; Schultze, W.; Mowitz, A.; Lindequist, U. Phytochemical Composition and in Vitro Antimicrobial and Antioxidant Activities of Essential Oils of Three Endemic Soqotraen Boswellia Species. Food Chem. 2011, 126, 6. [Google Scholar] [CrossRef]
- Camarda, L.; Dayton, T.; Di Stefano, V.; Pitonzo, R.; Schillaci, D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae). Ann. Chim. 2007, 97, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ho, C.T.; Chin, C.K.; Badmaev, V.; Ma, W.; Huang, M.T. Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med. 1998, 64, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Schillaci, D.; Cusimano, M.G.; Rishan, M.; Rashan, L. In Vitro Antimicrobial Activity of Frankincense Oils from Boswellia sacra Grown in Different Locations of the Dhofar Region (Oman). Antibiotics 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, S.; Young, G.; Oberg, C.; Nakaoka, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr. J. 2008, 23, 6. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Kamatou, G.P.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010, 76, 6. [Google Scholar] [CrossRef]
- El-Nagerabi, S.A.F.; Elshafie, A.E.; AlKhanjari, S.S.; Al-Bahry, S.N.; Elamin, M.R. Biological Activities of Boswellia Sacra Extracts on the Growth and Aflatoxins Secretion of Two Aflatoxigenic Species of Aspergillus Species. Food Control 2013, 34, 7. [Google Scholar] [CrossRef]
- Gotz, F. Staphylococci in colonization and disease: Prospective targets for drugs and vaccines. Curr. Opin. Microbiol. 2004, 7, 477–487. [Google Scholar] [CrossRef]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Wesenberg-Ward, K.E.; Tyler, B.J.; Sears, J.T. Adhesion and biofilm formation of Candida albicans on native and Pluronic-treated polystyrene. Biofilms 2005, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Di Stefano, V. In vitro anti-biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar] [CrossRef]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 2020, 82, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S. Acetyl-11-keto-beta-boswellic acid (AKBA); targeting oral cavity pathogens. BMC Res. Notes 2011, 4, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi Samani, M.; Mahmoodian, H.; Moghadamnia, A.; Poorsattar Bejeh Mir, A.; Chitsazan, M. The effect of Frankincense in the treatment of moderate plaque-induced gingivitis: A double blinded randomized clinical trial. Daru 2011, 19, 288–294. [Google Scholar] [PubMed]
- Bakhtiari, S.; Nematzade, F.; Hakemi-Vala, M.; Talebi, G. Phenotypic Investigation of the Antimicrobial Effect of Organic and Hydro-Alcoholic Extracts of Boswellia serrata on Oral Microbiota. Front. Dent. 2019, 16, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, S.; Hakemi-Vala, M.; Gholami, S. In vitro Antibacterial Effect of Hydroalcoholic Extract of Lawsonia inermis, Malva sylvestris, and Boswellia serrata on Aggregatibacter actinomycetemcomitans. Adv. Biomed. Res. 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Maraghehpour, B.; Khayamzadeh, M.; Najafi, S.; Kharazifard, M. Traditionally used herbal medicines with antibacterial effect on Aggegatibacter actinomycetemcomitans: Boswellia serrata and Nigella sativa. J. Indian Soc. Periodontol. 2016, 20, 603–607. [Google Scholar] [CrossRef]
- Winking, M.; Sarikaya, S.; Rahmanian, A.; Jodicke, A.; Boker, D.K. Boswellic acids inhibit glioma growth: A new treatment option? J. Neurooncol. 2000, 46, 97–103. [Google Scholar] [CrossRef]
- Ammon, H.P. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Knaus, U.; Wagner, H. Effects of boswellic acid of Boswellia serrata and other triterpenic acids on the complement system. Phytomedicine 1996, 3, 77–80. [Google Scholar] [CrossRef]
- Siemoneit, U.; Koeberle, A.; Rossi, A.; Dehm, F.; Verhoff, M.; Reckel, S.; Maier, T.J.; Jauch, J.; Northoff, H.; Bernhard, F.; et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br. J. Pharmacol. 2011, 162, 147–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, H.; Rashan, L.; Hassan, U.; Abbas, M.; Hakkim, F.L.; Green, I.R. Frankincense diterpenes as a bio-source for drug discovery. Expert Opin. Drug Discov. 2022, 17, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, P.; Eshete, A.; Sterck, F.J.; Zuidema, P.A.; Bongers, F. Limitations to sustainable frankincense production: Blocked regeneration, high adult mortality and declining populations. J. Appl. Ecol. 2011, 49, 164–173. [Google Scholar] [CrossRef]





| Current Scientific Name | Official Tree Name | Synonyms | Geographical Distribution | References |
|---|---|---|---|---|
| Boswellia sacra | Boswellia sacra Flueck. | Boswellia bhaw-dajiana Birdw. Boswellia bhaw-dajiana var. serrulata Engl. Boswellia carteri Birdw. Boswellia carteri var. Subintegra Engl. Boswellia carteri var. Undulatocrenata Engl. Boswellia undulatocrenata (Engl.) Engl. | Arabian Peninsula (Oman, Yemen) | [18] |
| Boswellia serrata | Boswellia serrata Roxb. Ex Colebr. | Boswellia balsamifera Spreng. Boswellia glabra Roxb. Boswellia thurifera Roxb. Ex Fleming Chloroxylon dupada Buch.-Ham. | India | [19] |
| - | Boswellia odorata Hutch. | - | Niger, northern Nigeria, and eastern Cameron | [20] |
| - | Boswellia popoviana Hepper | - | Socotra | [21] |
| - | Boswellia ruspoliana Engl. | - | Ethiopia, Somalia, Kenya | [22] |
| Boswellia socotrana | Boswellia socotrana Balf.f. | - | Socotra | [23] |
| - | Boswellia holstii Engl. | - | Ethiopia, Somalia, Kenya | [24] |
| - | Boswellia microphylla Chiov. | - | Ethiopia, Somalia, Kenya | [25] |
| - | Boswellia multifoliolata Engl. | - | Ethiopia, Somalia, Kenya | [26] |
| Boswellia nana | Boswellia nana Hepper | - | Socotra | [27] |
| Boswellia ogadensis | Boswellia ogadensis Vollesen | - | Ethiopia | [28] |
| Boswellia ovalifoliolata | Boswellia ovalifoliolata N.P.Balakr. & A.N.Henry | - | India | [29] |
| Boswellia pirottae | Boswellia pirottae Chiov. | - | Ethiopia | [30] |
| Boswellia ameero | Boswellia ameero Balf.f. | - | Socotra | [31] |
| - | Boswellia boranensis Engl. | - | Ethiopia, Somalia, Kenya | [32] |
| - | Boswellia bricchettii (Chiov.) Chiov. | - | [33] | |
| - | Boswellia bullata Thulin | - | Socotra | [34] |
| - | Boswellia chariensis Guillaumin | - | Ethiopia, Eritrea, Sudan | [35] |
| - | Boswellia dioscoridis Thulin | - | Socotra | [36] |
| Boswellia elongata | Boswellia elongata Balf.f. | - | Socotra | [37] |
| Boswellia frereana | Boswellia frereana Birdw. | - | Somalia | [38] |
| - | Boswellia globosa Thulin | - | Somalia | [39] |
| Boswellia dalzielii | Boswellia dalzielii Hutch. | - | Northern Nigeria | [40] |
| Ambilobea madagascariensis | Ambilobea madagascariensis (Capuron) Thulin, Beier & Razafim. | Boswellia madagascariensis Capuron | Madagascar | [41] |
| Garuga floribunda | Garuga floribunda Decne. | Boswellia javanica Turcz. Bursera javanica (Turcz.) Baill. Garuga abilo (Blanco) Merr. Garuga clarkii Merr. Garuga littoralis Merr. Garuga mollis Turcz. Garuga pacifica Burkill Garuga pinnata var. mollis Kurz | Southern China, Bhutan, Bangladesh, India, through southeast Asia to the Pacific Islands | [42] |
| Boswellia papyrifera | Boswellia papyrifera (Caill. Ex Delile) Hochst. | Amyris papyrifera Caill. ex Delile Boswellia occidentalis Engl. | Ethiopia | [43] |
| Boswellia rivae | Boswellia rivae Engl. | - | Ethiopia | [44] |
| Boswellia hildebrandtii | Boswellia hildebrandtii Engl. | - | Ethiopia, Somalia, Kenya | [45] |
| - | Boswellia elegans Engl. | - | Ethiopia, Somalia, Kenya | [46] |
| Boswellia neglecta | Boswellia neglecta S.Moore. | - | Eritrea | [47] |
| Frankincense Type | Country of Origin | Source | Chemical/Active Component | References |
|---|---|---|---|---|
| Boswellia dalzielii | Chad, Mali, Nigeria | Hydro-distilled leaf essential oil | δ-3-carene (27.7%), α-pinene (15.2%), p-cymene (9.5%), β-phellandrene (8.5%), isolongifolene (6.2%), and myrcene (5.7%). | [65] |
| Boswellia dalzielii | Nigeria | Hydro-distilled leaf essential oil | α-pinene (45.7%) and α-terpinene (11.5%), trans-sabinene hydrate (4.6%), cis-p-menth-2-en1-ol (2.9%), α-campholenal (2.7%), caryophyllene oxide, and α-phellandrene (2.3%) | [64] |
| B. carterii | Somalia | Gum resin | Esters (62.1%), 1-octyl acetate being predominant (60.0%). Alcohols amount to 15.4%, 1-octanol being the major component (12.7%), and diterpene constituents amount to 7.1%, including cembrene (1.4%), isocembrene (1.8%), incensole (2.7%), and isoincensole (0.8) and a mixture of monoterpene hydrocarbons which amounts to 9.9%. | [58] |
| B. rivae | Ethiopia | Essential oil from frankincense | α-Pinene (36.1–67.7%), δ -3-carene (12.2%), and limonene (12.0%). | [66] |
| B. neglecta | Ethiopia | Essential oil from frankincense | α-Pinene (36.1–67.7%), terpinen-4-ol (11.3%). | [66] |
| Microbe | Extract or Chemical Constituent | MIC (μg/mL) | References |
|---|---|---|---|
| Aggregatibacter actinomycetemcomitans ATCC 33384 (Gram-negative) | Boswellia serrata | 512 | [113] |
| Aggregatibacter actinomycetemcomitans JP2 NOV99 (Gram-negative) | HAE of Boswellia serrata | 78 | [112] |
| Streptococcus mutans ATCC 25175 (Gram-positive) | KBA AKBA BA ABA | 16 2 32 >128 | [109] |
| Streptococcus mutans PTCC 1688 (Gram-positive) | HAE of Boswellia serrata | 50,000 | [111] |
| Enterococcus faecalis ATCC 29212 (Gram-positive) | KBA AKBA BA ABA | 16 4 8 >128 | [109] |
| Enterococcus faecium ATCC 8042 (Gram-positive) | KBA AKBA BA ABA | 16 4 8 >128 | [109] |
| Actinomyces viscosus ATCC 15987 (Gram-positive) | KBA AKBA BA ABA | 8 2 64 >128 | [109] |
| Streptococcus sanguinis ATCC 10556 (Gram-positive) | KBA AKBA BA ABA | 8 2 128 >128 | [109] |
| Fusobacterium nucleatum ATCC 10953 (Gram-negative) | KBA AKBA BA ABA | >128 >128 >128 >128 | [109] |
| Prevotella intermedia ATCC 25611 (Gram-negative) | KBA AKBA BA ABA | 16 4 32 >128 | [109] |
| Porphyromonas gingivalis ATCC 33277 (Gram-negative) | KBA AKBA BA ABA | 8 4 32 >128 | [109] |
| Candida albicans PTCC 5027 | HAE of Boswellia serrata | 50,000 | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-da-Silva, C.L.C.; Sivakumar, N.; Asadi, H.; Chang-Chien, A.; Qoronfleh, M.W.; Ojcius, D.M.; Essa, M.M. Effects of Frankincense Compounds on Infection, Inflammation, and Oral Health. Molecules 2022, 27, 4174. https://doi.org/10.3390/molecules27134174
Almeida-da-Silva CLC, Sivakumar N, Asadi H, Chang-Chien A, Qoronfleh MW, Ojcius DM, Essa MM. Effects of Frankincense Compounds on Infection, Inflammation, and Oral Health. Molecules. 2022; 27(13):4174. https://doi.org/10.3390/molecules27134174
Chicago/Turabian StyleAlmeida-da-Silva, Cássio Luiz Coutinho, Nallusamy Sivakumar, Homer Asadi, Anna Chang-Chien, M. Walid Qoronfleh, David M. Ojcius, and Musthafa Mohamed Essa. 2022. "Effects of Frankincense Compounds on Infection, Inflammation, and Oral Health" Molecules 27, no. 13: 4174. https://doi.org/10.3390/molecules27134174